Abstract
Background:
The role of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) validated with video-assisted mediastinoscopic lymphadenectomy (VAMLA) for mediastinal restaging of patients with non-small cell lung cancer (NSCLC) after induction therapy has never been described.
Objective:
To report on our experience in this clinical setting.
Design:
Retrospective analysis of a prospectively built database.
Methods:
Patients with stage IIIA (N2) NSCLC who underwent EBUS-TBNA for mediastinal restaging after induction therapy were included. The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and diagnostic accuracy of EBUS-TBNA and VAMLA for mediastinal restaging were calculated. The number of patients needed to undergo confirmatory VAMLA (NNT) after a negative EBUS-TBNA for mediastinal restaging to avoid a case of pathologic (p) N2 disease after resection was also calculated.
Results:
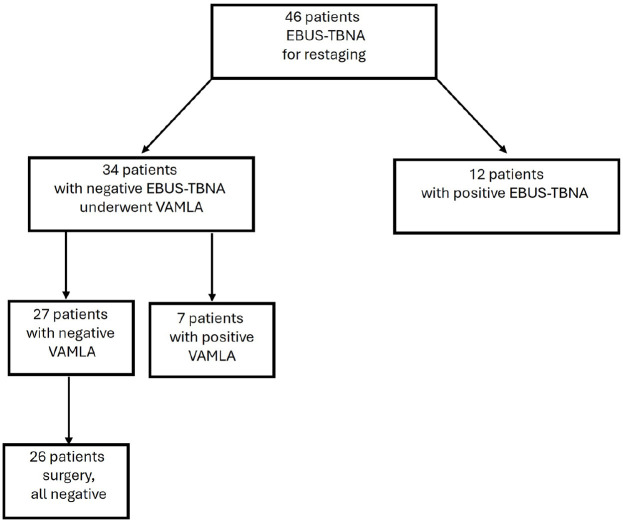
Forty-six patients underwent EBUS-TBNA which was positive in 12 patients and negative in 34. Patients with a negative EBUS-TBNA underwent VAMLA which was positive in seven cases. Of the other 27 patients with a negative VAMLA, 26 underwent resection that did not show N2 disease. The sensitivity, specificity, NPV, PPV, and diagnostic accuracy of EBUS-TBNA for restaging were 63.1%, 100%, 79.4%, 100%, and 84.7%, respectively. The sensitivity, specificity, NPV, PPV, and diagnostic accuracy of confirmatory VAMLA after EBUS-TBNA was 100%. The NNT confirmatory VAMLA after a negative EBUS-TBNA to avoid a case of pN2 disease at resection was five patients.
Conclusion:
EBUS-TBNA must remain as the first-choice test for invasive mediastinal restaging. However, the results of our study in terms of sensitivity and NPV, even considering the small size of our population, suggest that negative results of EBUS-TBNA should be interpreted with caution and surgical exploration of the mediastinum (specially VAMLA, if available) should be considered in these patients.
Keywords: EBUS-TBNA, lung cancer, lung cancer restaging, N2, restaging, VAMLA
Introduction
In patients with stage IIIA-N2 non-small cell lung cancer (NSCLC), current guidelines recommend definitive concurrent chemoradiation or neoadjuvant systemic therapy with or without radiotherapy. 1 Patients receiving neoadjuvant therapy with single station non-bulky N2 disease requiring only lobectomy may benefit from resection if mediastinal downstaging is achieved. The latest guidelines of the European Society of Thoracic Surgeons (ESTS) for preoperative mediastinal lymph node (LN) staging of NSCLC published in 2014 2 recommend using the same diagnostic techniques for restaging as for primary mediastinal staging. Thus, restaging should start with non-invasive image-based techniques such as computed tomography (CT) and positron emission tomography (PET) or combined PET/CT. If both imaging techniques do not show extrathoracic progression, their mediastinal findings should be confirmed by invasive techniques. While in primary staging minimally invasive endosonographic techniques, like endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and/or endoscopic ultrasound fine needle aspiration (EUS-FNA), are recommended as first invasive diagnostic tests, in restaging they are not recommended over surgical techniques given their low diagnostic accuracy.2 –4 Moreover, while in primary staging, in certain selected cases, confirmatory mediastinoscopy after a negative EBUS-TBNA could be spared before resection, in mediastinal restaging the ESTS guidelines recommend that all negative EBUS-TBNA results should be confirmed by surgical techniques before resection. The guidelines by the European Society of Gastrointestinal Endoscopy in collaboration with the European Respiratory Society and ESTS recommend using endosonography for mediastinal restaging after induction therapy with a grade C recommendation. 5 However, like in the ESTS guidelines, negative results should be confirmed by surgical techniques. Some authors 6 have questioned this approach based on the high negative predictive value (NPV) in their series; however, the NPV depends not only on the sensitivity of EBUS-TBNA but also on the prevalence of N2 after induction therapy. Moreover, although two previously published meta-analyses3,4 focused on the usefulness of endosonography for restaging reported the pooled sensitivity, the pooled NPV of EBUS-TBNA for mediastinal restaging was not described. Finally, although some of the previously published studies included patients who underwent confirmatory mediastinoscopy, the clinical usefulness of a confirmatory mediastinoscopy (measured as the number needed to treat (NNT: number of patients needed to undergo confirmatory mediastinoscopy to avoid a case of pathologic (p)N2 during resection after a negative EBUS-TBNA for mediastinal restaging)) has never been described in this clinical scenario.
The role of transcervical lymphadenectomies, like video-assisted mediastinoscopic lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA), as a single test or as a confirmatory procedure after a negative EBUS-TBNA for mediastinal restaging has been poorly described. To the best of our knowledge, only three studies performed by the same group7 –9 have focused on the role of TEMLA alone or in combination with EBUS-TBNA (alone and in combination with the esophageal approach using the same scope) for mediastinal restaging. In our institution mediastinal restaging after induction therapy is usually performed by EBUS-TBNA and negative results are confirmed by means of VAMLA. The aim of our study was to describe the diagnostic performance of EBUS-TBNA for mediastinal restaging after induction therapy and to estimate the clinical usefulness of confirmatory VAMLA after a negative EBUS-TBNA measured as NNT.
Methods
Study design and patients
The current study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 10 statement for observational studies. The checklist can be found as Supplemental Material (Supplemental File 1). We conducted a single-center study that consisted of a retrospective analysis of a prospectively built database. Without a previous calculation of sample size, we reviewed patients with pathologically proven stage IIIA-N2 single station NSCLC who underwent EBUS-TBNA for mediastinal restaging after induction therapy between June 2017 and December 2023. The inclusion criteria were patients with pathologically proven stage IIIA-N2 NSCLC who received at least three cycles of platinum-based chemotherapy with or without radiotherapy with or without immunotherapy, with stable disease or some response (as defined by response evaluation criteria in solid tumors (RECIST) 11 ). Patients with negative EBUS-TBNA underwent VAMLA and, if VAMLA was negative, lung resection with systematic nodal dissection (SND), which was considered the gold standard for the present study. Operability was assessed by medical history, physical examination, electrocardiogram, and pulmonary function tests. Patients with previous VAMLA, lung resection, and those with negative or suspicious EBUS-TBNA that could not undergo confirmatory VAMLA were excluded from the analysis. The Internal Review Board approved the study protocol (FAMT/P/23-149) and, given the retrospective design, patients’ informed consent was waived.
Endobronchial ultrasound-guided transbronchial needle aspiration
EBUS-TBNA was performed using a flexible bronchoscope (BFUC180F; Olympus Optical Co Ltd., Tokyo, Japan) with a distal probe capable of producing linear parallel scans of the mediastinal and peribronchial tissues and a working channel suited for the performance of TBNA under direct ultrasound guidance. General anesthesia was performed by an anesthesiologist using topical lidocaine spray and intravenous midazolam, propofol, and/or fentanyl according to standard recommendations 12 ; and patients were mechanically ventilated through a laryngeal mask. Contrary to primary staging, mediastinal re-staging consisted of the selective sampling of the positive lymph nodes (LNs) in the initial staging. LNs with a short-axis diameter of ⩾5 mm identified during the procedure were targeted under direct ultrasound visualization with a 21- or 22-gauge cytology needle specially designed for EBUS-TBNA (NA-201SX-4022; Olympus Optical Co Ltd.). The needle was guided beyond the bronchoscope channel and then pushed forward from the sheath to be inserted into the tracheal or bronchial wall under ultrasound guidance. Once the needle tip was inside the LN, negative pressure was maintained by a syringe at the proximal end of the catheter while the needle was pushed back and forth. Then, suction was released before removing the needle from the LN.
During the EBUS-TBNA procedure, all pathologically proven N2 mediastinal stations were sampled regardless of their size or their maximum standardized uptake value on the postinduction PET/CT. Negative nodal stations on EBUS-TBNA in the initial staging were not resampled except if they were suspicious on the postinduction PET/CT.
Pathologic examination
EBUS-TBNA samples were analyzed by a pathologist during a rapid on-site examination (Figure 1). During EBUS-TBNA, as many passes per LN as needed were obtained on request of the pathologist. For analysis purposes, EBUS-TBNA results were classified into two categories: negative (that included samples consistent with benignity (normal LN tissue/lymphocytes), atypical cells, necrotic tissue, or tissue non-representative of LN but with the absence of malignant cells) and positive (that included reliable malignant cells).
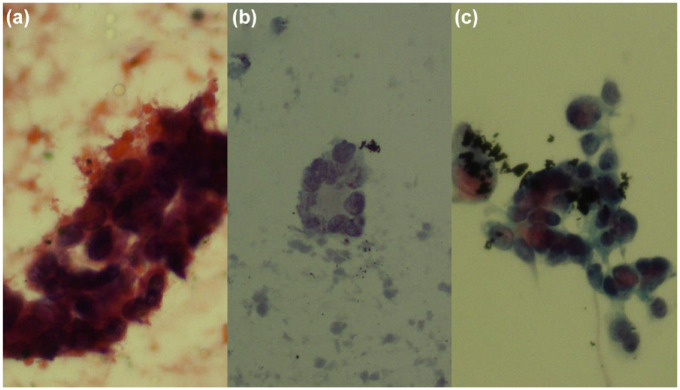
Figure 1.
Three cases of EBUS-TBNA: (a) Three-dimensional group of atypical epithelial cells were detected during ROSE (Diff Quick ×30), not in the paired Papanicolaou stained slide; VAMLA showed no malignancy, and it was considered as a true negative result of EBUS-TBNA. (b) Group of atypical cells (Papanicolaou ×20); VAMLA confirmed malignancy, and it was considered as a false negative result of EBUS-TBNA. (c) Group of definitive epithelial malignant cells (Papanicolaou ×20); no confirmatory test was necessary.
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; ROSE, rapid on-site evaluation; VAMLA, video-assisted mediastinoscopic lymphadenectomy.
Surgical staging
Every patient with a negative EBUS-TBNA underwent confirmatory VAMLA. The way VAMLA was performed has already been described elsewhere.13,14 First, the subcarinal nodal station was completely excised along the main bronchi, the pulmonary artery, and the esophagus. This dissection included the upper part of the para-esophageal nodes. Next, the superior vena cava and mediastinal pleura were exposed below the innominate artery, and the right paratracheal nodes, including the fatty tissue, were completely removed down to the azygos vein and right main bronchus. Finally, the left paratracheal nodes were carefully dissected and removed individually after identification of the left recurrent laryngeal nerve (Figure 2). In most cases, VAMLA was extended to hilar nodes at the main bronchi and the bronchus intermedius. For left-lung cancers, an extended cervical video-mediastinoscopy was added to VAMLA to explore and to take biopsies of the subaortic and paraaortic LNs. 15 SND was performed in patients undergoing resection following the recommendations of The Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pulmonology and Thoracic Surgery. 16 In brief, SND consisted of the excision of all LNs from hilar and mediastinal nodal stations ipsilateral to the primary tumor.
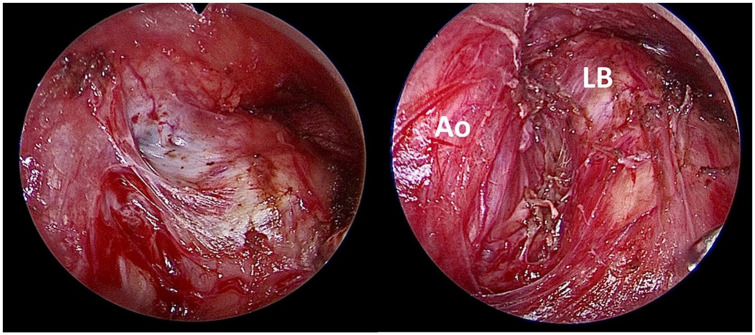
Figure 2.
A case of a 62-year-old woman with an NSCLC-NOS in the left upper lobe initially staged IIIB (cT4N2) by means of EBUS-TBNA (single N2a involvement at 4L nodal station). After induction chemoradiotherapy, the patient underwent PET/CT that showed a total metabolic response. An EBUS-TBNA was performed that didn’t show malignancy. A VAMLA was performed. The left photo shows the left inferior paratracheal space with a remnant of a lymph node (grayish area) with severe fibrosis, and the right photo shows the surgical bed after resection. No mediastinal involvement was detected in the surgical specimen.
Ao, aorta; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration; LB, left main bronchus; NSCLC-NOS, non-small cell lung cancer-not otherwise specified; VAMLA, video-assisted mediastinoscopic lymphadenectomy.
Statistical analysis
Data were entered into a database and analyzed using version 15 of STATA (StataCorp LLC, College Station, Texas, USA). Categorical variables were expressed as absolute and relative frequencies, continuous variables as means and standard deviations (SD) and non-normally distributed data as medians and interquartile ranges. The sensitivity, specificity, NPV, positive predictive value (PPV), and diagnostic accuracy of EBUS-TBNA for mediastinal restaging after induction therapy as well as VAMLA after a negative EBUS-TBNA result were calculated using the standard formulas. As a secondary outcome, the sensitivity, specificity, NPV, PPV, and diagnostic accuracy of PET/CT for mediastinal restaging after induction therapy were also calculated.
The number of patients needed to undergo confirmatory VAMLA (NNT) after a negative EBUS-TBNA for mediastinal restaging to avoid a case of pN2 disease after resection was also calculated. NNT was estimated as: 1/ARR (absolute risk reduction), where ARR is CER (Control Event Rate) − EER (Experimental Event Rate), considering CER as the rate of pN2 disease after resection if all patients underwent resection without a confirmatory VAMLA; and EER the rate of pN2 disease in patients undergoing resection after confirmatory VAMLA.
Results
Forty-six patients met the inclusion criteria. Table 1 shows patients’ characteristics. There were 35 men and 11 women with a mean age of 61.8 years (SD ±6.6). All patients had single station N2 involvement (N2a), mainly previously diagnosed by EBUS-TBNA (n = 43; 93.5%). The right lower paratracheal (4R) nodal station was the most frequently involved station (22 [47.9%]), and adenocarcinoma 25 (54.3%) was the most frequent histological type. Most patients (n = 42; 91.3%) underwent platinum-based induction chemoradiotherapy at full dose (mean 58.3 Gy ± 3.9); and 4 (8.5%) underwent induction immunotherapy preceded by chemoradiotherapy or chemotherapy. The mean time between the end of induction therapy and EBUS-TBNA was 5.1 (±1.7) weeks.
Table 1.
Patient’s characteristics (N = 46).
| Age (m, ±SD) | 61.8 ± 6.6 years | |
|---|---|---|
| Sex (n, %) | Women 11 (23.9) | |
| Men 35 (76.1) | ||
| Histological type (n, %) | NSCLC-NOS 5 (10.9) | |
| Neuroendocrine 1 (2.1) | ||
| Squamous cell carcinoma 15 (32.7) | ||
| Adenocarcinoma 25 (54.3) | ||
| Tumor location (n, %) | Right upper lobe 20 (43.5) | |
| Middle lobe 2 (4.3) | ||
| Right lower lobe 12 (26.1) | ||
| Left upper lobe 8 (17.4) | ||
| Left lower lobe 4 (8.7) | ||
| N2 disease location | Subcarinal 16 (34.7) | |
| Right lower paratracheal 22 (47.9) | ||
| Left lower paratracheal 8 (17.4) | ||
| Previous diagnostic method | Mediastinoscopy 3 (6.5) | |
| EBUS-TBNA 43 (93.5) | ||
| Induction therapy (n, %) | Chemoradiotherapy 42 (91.3) | Cisplatin etoposide 1 |
| Cisplatin vinorelbine 30 | ||
| Carboplatin vinorelbine 11 | ||
| Chemoradiotherapy + immunotherapy 3 (6.5) | ||
| Chemotherapy + immunotherapy 1 (2.2) | ||
| Radiotherapy dose (m, ±SD) | 58.3 Gy ±3.9 | |
| Time between end of induction therapy and EBUS-TBNA (m, ±SD) | 5.1 weeks ±1.7 | |
| Time between EBUS-TBNA and resection (m, ±SD) | 34.6 days ±11.2 | |
| Number of mediastinal stations sampled during EBUS-TBNA (m, ±SD) | 1.22 ± 0.44 | |
| Number of passes during EBUS-TBNA (m, ±SD) | 5.1 ± 2.4 | |
| Number of lymph nodes dissected during VAMLA (m, ±SD) | 11.2 ± 6.2 | |
| Number of mediastinal stations dissected during VAMLA (m, ±SD) | 3 ± 0.63 | |
| Resection surgery (n, %) (N = 26) | Sublobar resection 2 (7.7) | |
| Lobectomy 20 (76.9) | ||
| Bilobectomy 4 (15.4) | ||
| Final N status (n, %) | Persistent N2 disease 19 (41.3) | |
| Downstaging 27 (58.7) | ||
| Time between EBUS-TBNA and VAMLA (m, ±SD) | 20.3 days ±8.3 | |
| Time between EBUS-TBNA and resection (m, ±SD) | 34.6 days ±11.2 | |
| Time between VAMLA and resection (m, ±SD) | 14.3 days ±8.3 | |
| Operative time during resection after induction (m, ±SD) | 203 min ±49.8 | |
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; m, mean; n, number; NSCLC-NOS, non-small cell lung cancer-not otherwise specified; SD, standard deviation; VAMLA, video-assisted mediastinoscopic lymphadenectomy.
EBUS-TBNA showed a positive result in 12 patients (Figure 3). EBUS-TBNA was negative in 34 patients, and all of them underwent confirmatory VAMLA, which found N2 disease in seven patients. Of the remaining 27 patients with a negative VAMLA, 26 patients underwent resection; 1 was excluded from resection due to his worsening clinical condition (he suffered from radiation pneumonitis). After resection with SND 11 (42.3%) patients presented complete pathologic response (Table 2), and all patients showed N0 disease. No complications related to EBUS-TBNA or VAMLA were registered.
Figure 3.
Patients’ flowchart.
Table 2.
Tumor status.
| cTN (N = 46; n, %) | ycTN (N = 46; n (%)) | ypTN (N = 26; n (%)) |
|---|---|---|
| T1N2 12 (26.1) | T1N0 14 (30.4) | T0N0 11 (42.3) |
| T2N2 13 (28.3) | T2N0 10 (21.7) | |
| T3N2 14 (30.4) | T3N0 3 (6.5) | T1N0 14 (53.9) |
| T4N2 7 (15.2) | T1N2 6 (13) | |
| T2N2 8 (17.5) | T1N1 1 (3.8) | |
| T3N2 5 (10.9) |
cTN, clinical TN classification; ycTN, clinical TN classification after induction therapy; ypTN, pathologic TN classification after induction therapy.
The prevalence of N2 disease in our series was 41.3% (19 out of 46 patients). The sensitivity, specificity, NPV, PPV, and diagnostic accuracy of PET/CT for restaging after induction therapy in our series were 11.1%, 85.7%, 24%, 33.3%, and 56.5%, respectively. The sensitivity, specificity, NPV, PPV, and diagnostic accuracy of EBUS-TBNA for restaging after induction therapy in our series were 63.1%, 100%, 79.4%, 100%, and 84.7%, respectively. The sensitivity, specificity, NPV, PPV, and diagnostic accuracy of confirmatory VAMLA after EBUS-TBNA for restaging after induction therapy in our series were 100%. The NNT of confirmatory VAMLA after a negative EBUS-TBNA for restaging to avoid a case of pN2 disease at resection was five patients.
Discussion
In our study EBUS-TBNA attained a good sensitivity for mediastinal restaging after induction therapy. However, all negative results had to be surgically confirmed given that NPV was not reliable enough. The combination EBUS-TBNA plus confirmatory VAMLA attained an optimal diagnostic performance with a low NNT.
While there is abundant evidence supporting the use of EBUS-TBNA for primary mediastinal staging of NSCLC, studies focused on restaging after induction therapy are limited. Two meta-analyses reviewing the usefulness of endosonography for restaging have been published.3,4 One of them included four studies6,7,17,18 of patients undergoing exclusively EBUS-TBNA for restaging, while the other included six studies7 –9,17 –19 (five exclusively performing EBUS-TBNA and one study with EUS(B)-FNA used in combination with EBUS-TBNA). Both meta-analyses included 261 and 439 patients, respectively, and reported the same pooled sensitivity (65%) with a similar pooled specificity (98% and 99%, respectively). However, in both cases, the variability for sensitivity was high (I2 of 79.1% and 79.7%, respectively) with sensitivity values ranging from 40% to 82%. In both meta-analyses, the prevalence of N2 disease and the NPV of the procedures were not described. Nonetheless, the variability of prevalence and NPV, although not described, maybe even higher. They can be calculated from the reported data and ranged from 18.3% to 94.3% for prevalence, and from 20% to 88% for NPV between series. Therefore, although Nasir et al. 6 suggested in their study that confirmatory mediastinoscopy could be spared after a negative EBUS-TBNA for restaging, this message should be interpreted with caution and only considering their results: the best in terms of NPV (88%) with the lowest in prevalence of N2 (18.3%). In this setting, while two recent publications20,21 have increased the debate on the usefulness of confirmatory mediastinoscopy after negative EBUS-TBNA in primary staging, in the restaging setting there is no doubt about the need for confirmatory surgical exploration. Actually, in our study, with a prevalence of N2 disease of 41.3% and an NPV of 79.4%, the NNT of confirmatory VAMLA was low (five patients). Unfortunately, not all studies focused on EBUS-TBNA restaging included confirmatory mediastinoscopy and those with a confirmatory test did not report the NNT. However, in studies where patients underwent confirmatory mediastinoscopy or TEMLA,6 –8,18 the rate of positivity was 22% and 66% for mediastinoscopy6,18 and 21% and 27% for TEMLA.7,8 These results emphasize the message that confirmatory surgical tests after negative EBUS-TBNA in restaging should be maintained.
Current guidelines1,2,5 recommend starting primary mediastinal staging by means of endosonography. In patients with N2 diagnosed by endosonography, restaging can be performed again with endosonography, reserving mediastinoscopy for confirmation in case of a negative endosonography. In our institution, primary staging starts with EBUS-TBNA, with VAMLA as a confirmatory test in cases of negative EBUS-TBNA. When EBUS-TBNA detects N2 disease, restaging is performed by means of EBUS-TBNA and negative results are always confirmed by means of VAMLA. In case that N2 disease is diagnosed by means of VAMLA during primary staging, restaging is exclusively based on PET/CT because no nodal tissue remains in the nodal stations dissected with VAMLA. Although the results of our series should be considered with caution given the sample size, the persistence of N2 disease in our series was 41.3% and the combination of EBUS-TBNA plus VAMLA diagnosed all patients with persistent N2 disease before surgical resection. Thus, the combination of EBUS-TBNA plus VAMLA attained an optimal diagnostic performance in our series.
To the best of our knowledge, the performance of pathological restaging in daily practice between institutions has not been reported. However, it does not seem to be globally performed because the NCCN guidelines 1 affirm that the majority of NCCN Member Institutions do not pathologically restage mediastinal LNs after induction therapy and prior to surgery. This rate could increase after the promising results of pathological response of the recently published randomized controlled trials (RCTs) on immunotherapy in patients with resectable tumors (KEYNOTE-671 and CHECKMATE 816).22,23 However, there are several elements to consider in this regard. First, in both RCTs invasive restaging was not performed since in both studies the protocol was not adherent with the current guidelines not only in the restaging but also in the primary mediastinal staging. Second, although major and complete pathological responses were described, no studies reported the rate of mediastinal downstaging in patients with N2 disease at the time of randomization and, therefore, it is unclear if these patients could benefit or not from invasive restaging (for instance, with a mediastinal downstaging rate of 99% the usefulness of restaging in these patients should be arguable). Third, the mediastinal restaging of patients receiving immunotherapy based only on PET/CT is challenging, given that, contrary to chemotherapy, immunotherapy produces responses that are not manifested with a decrease in size and metabolic activity; thus, RECIST criteria are not applicable and objective tumor response has to rely on other PET/CT features. 24 Finally, to the best of our knowledge, there is not any currently published series of invasive restaging exclusively of patients receiving immunotherapy to recommend avoiding invasive restaging in this population. In our series, four patients underwent induction immunotherapy in the setting of RCT. After induction, three presented persistent nodal disease diagnosed by means of EBUS-TBNA, while the other presented downstaging (negative EBUS-TBNA confirmed by means of VAMLA and resection). The only patient who presented downstaging had a response on PET/CT, while the others did not have any changes on PET/CT, although in their initial PET/CT, the mediastinum was normal. Unfortunately, in our series, patients who received immunotherapy were only four and that does not allow us to compare the diagnostic performance of EBUS-TBNA with those who received chemotherapy.
Our study has two main limitations: the retrospective design and the small series, which may affect the statistical significance of our results. However, these characteristics are similar to previous studies (all retrospective and similar in size). On the contrary, as a strength, our study has been performed in a highly specialized center for both EBUS-TBNA and VAMLA.
Conclusion
In conclusion, EBUS-TBNA should remain the first choice in invasive mediastinal restaging after induction therapy. However, the results of our study in terms of sensitivity and NPV, even considering the small size of our population, suggest that negative results of EBUS-TBNA should be interpreted with caution and surgical exploration of the mediastinum (specially VAMLA, if available) should be considered in these patients.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666241301284 for Endobronchial ultrasound-guided transbronchial needle aspiration validated with video-assisted mediastinoscopic lymphadenectomy in the mediastinal restaging of patients with stage IIIA non-small cell lung cancer after induction therapy by Bruno García-Cabo, Nina Reig, Ramón Rami-Porta, Sergi Call, Lluís Esteban, Bienvenido Barreiro, Efraín Reyes, Carme Obiols, Juan Manuel Ochoa, Xavier Morlius, Xavier Tarroch, Mireia Serra and José Sanz-Santos in Therapeutic Advances in Respiratory Disease
Acknowledgments
Not applicable.
Footnotes
ORCID iD: José Sanz-Santos  https://orcid.org/0000-0001-8354-414X
https://orcid.org/0000-0001-8354-414X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bruno García-Cabo, Pulmonology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain; Department of Medicine, School of Medicine and Health Sciences, University of Barcelona, Terrassa, Barcelona, Spain.
Nina Reig, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Ramón Rami-Porta, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain; Network of Centers for Biomedical Research on Respiratory Diseases (CIBERES), Lung Cancer Group, Terrassa, Barcelona, Spain.
Sergi Call, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain; Department of Morphological Sciences, Medical School, Autonomous University of Barcelona, Bellaterra, Barcelona, Spain.
Lluís Esteban, Pulmonology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Bienvenido Barreiro, Pulmonology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Efraín Reyes, Pulmonology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Carme Obiols, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Juan Manuel Ochoa, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Xavier Morlius, Pathology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Xavier Tarroch, Pathology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
Mireia Serra, Thoracic Surgery Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Terrassa, Barcelona, Spain.
José Sanz-Santos, Pulmonology Department, Hospital Universitari Mútua Terrassa, University of Barcelona, Plaça Dr Robert 5, Terrassa, Barcelona 08221, Spain; Department of Medicine, School of Medicine and Health Sciences, University of Barcelona, Terrassa, Barcelona, Spain.
Declarations
Ethics approval and consent to participate: The Internal Review Board (Comité de Ética de la Investigación con Medicamentos de la Fundació Assistencial Mútua Terrassa) approved the study protocol (registry number FAMT/P/23-149) and, given the retrospective design, patients’ informed consent was waived.
Consent for publication: The Internal Review Board (Comité de Ética de la Investigación con Medicamentos de la Fundació Assistencial Mútua Terrassa), considering the retrospective design and confidentiality of patients’ data, waived patients’ informed consent.
Author contributions: Bruno García-Cabo: Conceptualization; Data curation; Formal analysis; Investigation; Writing – original draft.
Nina Reig: Formal analysis; Investigation; Writing – original draft.
Ramón Rami-Porta: Data curation; Investigation; Methodology; Writing – original draft.
Sergi Call: Formal analysis; Investigation; Methodology; Writing – original draft.
Lluís Esteban: Investigation; Methodology; Writing – review & editing.
Bienvenido Barreiro: Investigation; Writing – review & editing.
Efraín Reyes: Investigation; Writing – review & editing.
Carme Obiols: Investigation; Writing – review & editing.
Juan Manuel Ochoa: Investigation; Writing – review & editing.
Xavier Morlius: Investigation; Writing – review & editing.
Xavier Tarroch: Investigation; Writing – review & editing.
Mireia Serra: Investigation; Writing – review & editing.
José Sanz-Santos: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. National Comprehensive Cancer Network. Non-small cell lung cancer (Version 2.2024), https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2024, accessed 5 March 2024).
- 2. De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 787–798. [DOI] [PubMed] [Google Scholar]
- 3. Muthu V, Sehgal IS, Dhooria S, et al. Efficacy of endosonographic procedures in mediastinal re-staging of lung cancer after neoadjuvant therapy: a systematic review and diagnostic accuracy meta-analysis. Chest 2018; 154(1): 99–109. [DOI] [PubMed] [Google Scholar]
- 4. Jiang L, Huang W, Liu J, et al.; AME Lung Cancer Collaborative Group. Endosonography with lymph node sampling for restaging the mediastinum in lung cancer: a systematic review and pooled data analysis. J Thorac Cardiovasc Surg 2020; 159(3): 1099–1108.e5. [DOI] [PubMed] [Google Scholar]
- 5. Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015; 46(1): 40–60. [DOI] [PubMed] [Google Scholar]
- 6. Nasir BS, Bryant AS, Minnich DJ, et al. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014; 98(3): 1008–1012. [DOI] [PubMed] [Google Scholar]
- 7. Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultra-sound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy—a prospective study. Eur J Cardiothorac Surg 2010; 37(5): 1180–1184. [DOI] [PubMed] [Google Scholar]
- 8. Szlubowski A, Zieliński M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014; 46(2): 262–266. [DOI] [PubMed] [Google Scholar]
- 9. Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013; 8(5): 630–636. [DOI] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, et al.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18(6): 800–804. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 12. Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68: i1–i44. [DOI] [PubMed] [Google Scholar]
- 13. Witte B, Hürtgen M. Video-assisted mediastinoscopic lymphadenectomy. Multimed Man Cardiothorac Surg 2007; 2007(1018): mmcts.2006.002576. [DOI] [PubMed] [Google Scholar]
- 14. Call S, Obiols C, Rami-Porta R, et al. Video-assisted mediastinoscopic lymphadenectomy for staging non-small cell lung cancer. Ann Thorac Surg 2016; 101: 1326–1333. [DOI] [PubMed] [Google Scholar]
- 15. Obiols C, Call S, Rami-Porta R, et al. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2012; 41:1043–1046. [DOI] [PubMed] [Google Scholar]
- 16. The Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery. Intraoperative lymph node staging in bronchogenic carcinoma surgery. Consensus Report. Arch Bronconeumol 2001; 37: 495–503. [PubMed] [Google Scholar]
- 17. Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008; 26(20): 3346–3350. [DOI] [PubMed] [Google Scholar]
- 18. Cetinkaya E, Usluer O, Yılmaz A, et al. Is endobronchial ultrasound-guided transbronchial needle aspiration an effective diagnostic procedure in restaging of non-small cell lung cancer patients? Endosc Ultrasound 2017; 6(3): 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon HY, Lee JC, Kim SW, et al. Prognosis of multi-level N2-positive non-small cell lung cancer according to lymph node staging using endobronchial ultra-sound-transbronchial biopsy. Thorac Cancer 2018; 9(6): 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bousema JE, Dijkgraaf MGW, van der Heijden EHFM, et al. Endosonography with or without confirmatory mediastinoscopy for resectable lung cancer: a randomized clinical trial. J Clin Oncol 2023; 41(22): 3805–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanz-Santos J, Almagro P, Malik K, et al. Confirmatory mediastinoscopy after negative endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal staging of lung cancer: systematic review and meta-analysis. Ann Am Thorac Soc 2022; 19: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 22. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386: 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 2023; 389: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aide N, Hicks RJ, Le Tourneau C, et al. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging 2019; 46(1): 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666241301284 for Endobronchial ultrasound-guided transbronchial needle aspiration validated with video-assisted mediastinoscopic lymphadenectomy in the mediastinal restaging of patients with stage IIIA non-small cell lung cancer after induction therapy by Bruno García-Cabo, Nina Reig, Ramón Rami-Porta, Sergi Call, Lluís Esteban, Bienvenido Barreiro, Efraín Reyes, Carme Obiols, Juan Manuel Ochoa, Xavier Morlius, Xavier Tarroch, Mireia Serra and José Sanz-Santos in Therapeutic Advances in Respiratory Disease