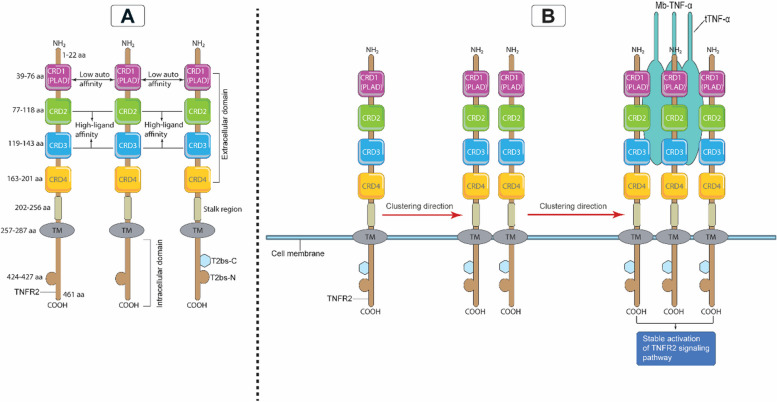
Fig. 3.
TNFR2 protein structure and assembly on the cell surface. A. The full amino acid protein structure of the TNFR2 extracellular and intracellular domains. In the extracellular amino N-terminus domain, the organization of each CRD (1–4) is indicated, with the CRD1 harboring the self-structure interacting domain PLAD that interacts with low affinity with TNF-α and the regions of the CRD2 and CRD3 interacting with strong affinity with TNF-α. In the intracellular C-terminus domain, the receptor contains the TRAF binding sites (T2bs-C and T2bs-N) that actively recruit TRAF2 to inhibit or initiate signaling. B. A single chain of TNFR2 will start clustering with a second TNFR2 chain via their PLADs, then the two chains will cluster with a third chain via PLAD-PLAD interactions; then a Mb-TNF-α trimer binds to these clustering chains of TNFR2 to form a fully stable and active signalosome. CRD, cysteine-rich domain; PLAD, pre-ligand assembly domain; TNFR2, tumor necrosis receptor type two; Mb-TNF-α, membrane-bound tumor necrosis factor; TRAF, TNF receptor associated factor; T2bs-C, TRAF2-binding site C; T2bs-N, TRAF2-binding site N