Abstract
Toxoplasmosis, a parasitic disease, can cause fatal multi-organ failure in immunocompromised patients. The lack of specificity in the symptoms and the need to confirm a diagnosis of tachyzoites in fluids or tissues through microscopic examination leads to a delay in reaching a diagnosis. A 28-year-old woman with severe aplastic anemia received stem cell transplantation seven months ago, presented with fever. Computed Tomography scan and ultrasonography showed moderate pleural, pericardial, peritoneal, and pelvic effusions. Metagenomic next-generation sequencing of blood and alveolar lavage fluid was done, 11,082 and 17,154 sequence readings of Toxoplasma gondii were detected, accounting for 1.34% and 17.09% of genome coverage, respectively. Then, marrow aspirate smears showed Toxoplasma gondii tachyzoites and pseudocyst. This case report alerts clinicians about Toxoplasma gondii infection in stem cell transplantation patients with multiple serous effusions and fever. Clinical trial: Not applicable.
Keywords: Toxoplasma gondii, Stem cell transplantation, Multiple serous effusions, Severe aplastic anemia
Introduction
Toxoplasmosis is a parasitic disease that can cause fatal multi-organ failure in immunocompromised patients [1–3]. Stem cell transplantation patients routinely receive trimethoprim-sulfamethoxazole (SMZ) for prophylaxis and have a lower risk of Toxoplasma gondii infection, especially those with toxoplasma-seronegative recipients and donors [4]. However, once ignored, it can be fatal. Here we present a case of a patient with disseminated toxoplasmosis with moderate pleural, pericardial, peritoneal, and pelvic effusions.
Case presentation
A 28-year-old female with severe aplastic anemia underwent haploidentical stem cell transplantation seven months ago, presented with fever for four days without cough, sputum, shortness of breath. Physical examination showed anemia, coarse breathing sounds in both lungs, and no dry or wet rales heard. Continuous oral administration of cyclosporine A (begining with 3 mg/kg.d and dosage ajusted according to the concentration of cyclosporine A), acyclovir (0.8 g/day), and trimethoprim-sulfamethoxazole (1.92 g/day) is recommended for conditioning in order to prevent graft-versus-host disease, viruses, pneumocystis pneumonia, and toxoplasmosis infection. She lived in a city in Shaanxi province, China. She gave no history of recent travel and no history of pet ownership. The donor and recipient had negative IgG antibody of Toxoplasma gondii serological tests before transplantation.
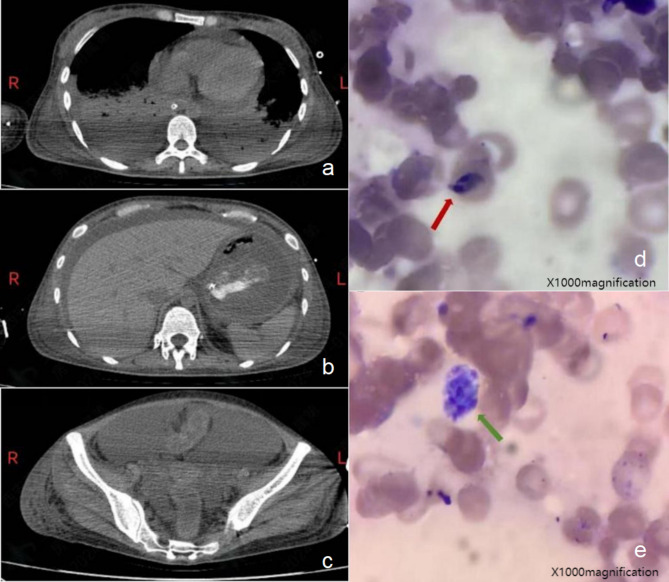
A computed tomography of the head, chest, abdomen, and pelvis showed pleural, pericardial, peritoneal, and pelvic effusions (Fig. 1a and c). Laboratory investigations were showed in Table 1.
Fig. 1.
Imaging and pathology findings. (a) Computed tomography (CT) of the thoracic cavity and moderate pleural and pericardial effusion. (b) CT of abdominal cavity and peritoneal effusion. (c) CT of pelvic cavity and pelvic effusion. (d) Bone marrow smear of Toxoplasma gondii tachyzoites (Red arrow, wright staining,×1000magnification). (e) Bone marrow smear of Toxoplasma gondii pseudocyst (Green arrow, wright staining,×1000magnification)
Table 1.
Laboratory findings and their respective reference values
| Test | Result | Reference Range |
|---|---|---|
| Hemoglobin | 6.2 g/dL | 14 to 18 g/dL |
| Absolute neutrophil count | 0.64 × 109/L | 2.5 to 7 × 109/L |
| Platelets | 43 × 109/L | 150 to 400 × 109/L |
| Albumin | 21.5 g/L | 40 to 55 g/L |
| Prothrombin time | 14.7s | 11–14 s |
| Activated partial thrombin time | 47.5s | 28–45.3 s |
| Fibrinogen | 1.96 g/L | 2–4 g/L |
| D-Dimer | 9.99 mg/L | 0-1 mg/L |
| Fibrinogen degradation products | 20.7.mg/L | 0-5 mg/L |
| Erythrocyte sedimentation rate | 23 mm/h | 0–20 mm/h |
| 1-3-β-D glucan | < 10pg/ml | < 60pg/ml |
| Galactomannan | 0.15 µg/L | < 0.5 µg/L |
| Procalcitonin | 2.8ng/mL | < 0.05ng/mL |
| Pro-Brain natriuretic peptide | 7723pg/mL | < 125pg/mL |
| Cardiac troponin I | 2126.17pg/mL | 24-30pg/mL |
| Cardiac troponin T | 1.120ng/mL | 0 to 0.01ng/mL |
| Glutamic-pyruvic transaminase | 147U/L | 7 to 40U/L |
| Aspartate transaminase | 275U/L | 13 to 35U/L |
| Alkaline phosphatase | 313U/L | 35-100U/L |
| Total bilirubin | 99.5µmol/L | 3.4–17.1µmol/L |
| Direct bilirubin | 83.7µmol/L | 0-3.4µmol/L |
| Urea nitrogen | 16.08mmol/L | 1.8 to 7.1mmol/L |
| Creatinine | 190µmol/L | 61.9 to 114.9µmol/L |
| Serum amylase | 230U/L | 40 to 140U/L |
Aerobic and anaerobic blood bacterial culture, as well as fungal culture was negative. Metagenomic next-generation sequencing (mNGS, NextSeq CN500,high throughput DNA sequencing) of blood and alveolar lavage fluid was done, and 11,082 and 17,154 sequence readings of Toxoplasma gondii were detected and accounting for 1.34% and 17.09% of genome coverage, respectively. One day later, bone marrow aspiration was done and bone marrow smears, with wright staining showed Toxoplasma gondii tachyzoites (Fig. 1d, red arrow) and pseudocyst (Fig. 1e, green arrow). Diagnosis of toxoplasmosis was confirmed.
Discussion
Common presentations of toxoplasmosis include fever, encephalopathy, and pneumonia [5]. The symptoms of this patient included fever and multiple serosal cavity effusions, which could easily lead to a misdiagnosis as ordinary parapneumonic effusion and hypoproteinemia [6]. However, the speculation is that the heart, pancreas, lungs, bone marrow, liver, and kidneys were all involved, indicating disseminated toxoplasmosis. Febrile pancytopenia may be a clinical manifestation of disseminated toxoplasmosis. The other frequently involved organs include eyes, heart, liver, pancreas, bone marrow, bladder, lymph nodes, kidney, spleen, and skin. The lack of specificity in the symptoms often leads to a delay in diagnosis [7]. Therefore the specific symptoms presented in this patient were not noticed as toxoplasmosis at first.
A timely and accurate diagnosis of toxoplasmosis is critical. Post-transplantation toxoplasma serology is unreliable because of profound immunosuppression. PCR-based testing has become the preferred method for diagnosis [8, 9]. Confirmation of the diagnosis is provided by the demonstration of tachyzoites in fluids or tissues by microscopic examination [10]. Although a direct examination of tachyzoites is the fastest and cheapest means of diagnosis, it frequently lacks sensitivity. Metagenomic next-generation sequencing of bronchoalveolar lavage fluid, blood, bone marrow aspirate, and cerebrospinal fluid may give clues for early diagnosis and avoid missed diagnosis [11]. For this patient, Toxoplasma gondii was identified in peripheral blood and alveolar lavage fluid by metagenomic next-generation sequencing. Then, marrow aspirate smears showed Toxoplasma gondii tachyzoites and pseudocyst.
For stem cell transplantation, patients with toxoplasmosis should be started as soon as possible with first-line medications, including pyrimethamine, sulfadiazine, and leucovorin [12]. This patient was infected based on oral trimethoprim-sulfamethoxazole prevention, and it may be related to poor drug enteric absorption. Intravenous trimethoprim-sulfamethoxazole was given once diagnosed.
While prophylactic agents are effective in reducing the risk of toxoplasmosis, certain patients may still experience reactivation of latent infection or primary infection or infection from blood transfusion because of incomplete suppression of the parasite [5]. This can occur because of drug resistance, suboptimal dosing, or altered pharmacokinetics in patients with compromised immune systems [13], such as those undergoing organ transplantation. When toxoplasmosis develops despite prophylaxis, it often presents more aggressively, leading to complications like toxoplasmic encephalitis or disseminated infection. These cases are associated with high morbidity and mortality [14], requiring prompt diagnosis and intensified treatment regimens. The occurrence of toxoplasmosis in this context underscores the importance of close clinical monitoring and possibly adjusting prophylactic strategies.
Unfortunately, the patient died of multiple organ failure Toxoplasma gondii.
This report has several limitations. First, pleural, pericardial, peritoneal, and pericardial effusions were not pathologically tested for. We could not confirm that damage to pancreas and liver and myocarditis was caused by Toxoplasma gondii infection. Second, we did not detect the concentration of trimethoprim- sulfamethoxazole and could not explain why we failed to prevent the Toxoplasma gondii infection for this patient.
Conclusion
This case underscores the importance of considering Toxoplasma gondii infection in stem cell transplantation patients presenting with multiple serous effusions and fever. While a single case cannot represent the broader population, it highlights the need for careful monitoring and a high index of suspicion in at-risk patients. Early diagnosis, potentially through metagenomic next-generation sequencing, may improve outcomes by facilitating timely and targeted treatment of toxoplasmosis.
Abbreviations
- SMZ
Trimethoprim-sulfamethoxazole
- CT
Computed tomography
- mNGS
Metagenomic next-generation sequencing
Author contributions
Xiaoning wang, writing original draft. Hao Li and Le Ma, Data collection. Zhao Jing and Zhang Mei, investigation. Fatahichegeni Mahsa and Ansarian Mohammad Amin edited the manuscript. Pengcheng He, Design and data analysis. All authors revised and approved the final manuscript.
Funding
Project of Clinical Research Center of Xi’an Jiaotong University (2023-XKCRC-06) and National Key R&D Program (grant no. 2022YFC2502700).
Data availability
The datasets used may be made available by the corresponding author upon reasonable request.
Declarations
Ethics approval
The study was approved by the ethical review boards of the first affiliated hospital of Xi’an jiaotong university.
Consent for publication
Written informed consent for publication was obtained from the patient’s husband.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montoya JG, Gomez CA. Toxoplasmosis after solid organ transplantation. Transpl Infections: Fourth Ed 2016:781–93.
- 2.Asensi Canto P, Mayordomo E, Dorado A, Villalba M, Mañez RB, González E, Salavert M, Facal A, Chorão P, Balaguer A. Disseminated toxoplasma infection after hematopoietic stem cell transplantation with myositis and encephalitis. Transpl Infect Disease. 2023;25(4):e14067. 10.1111/tid.14067. [DOI] [PubMed] [Google Scholar]
- 3.Zhai W, Zhang L, Wang J, He Y, Jiang E, Feng S, Han M. Toxoplasma Gondii infection after allogeneic hematopoietic stem cell transplantation in patients with hematological diseases: 2 cases report and literature reviews. Zhonghua xue ye xue Za Zhi = Zhonghua Xueyexue Zazhi. 2023;44(10):861–3. 10.3760/cma.j.issn.0253-2727.2023.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aerts R, Mehra V, Groll AH, Martino R, Lagrou K, Robin C, Perruccio K, Blijlevens N, Nucci M, Slavin M. Guidelines for the management of Toxoplasma gondii infection and disease in patients with haematological malignancies and after haematopoietic stem-cell transplantation: guidelines from the 9th European Conference on Infections in Leukaemia, 2022. The Lancet Infectious Diseases 2024;24(5):e291-e306. 10.1016/S1473-3099(23)00495-4 [DOI] [PubMed]
- 5.Layton J, Theiopoulou D-C, Rutenberg D, Elshereye A, Zhang Y, Sinnott J, Kim K, Montoya JG, Contopoulos-Ioannidis DG. Clinical spectrum, radiological findings, and outcomes of severe toxoplasmosis in immunocompetent hosts: a systematic review. Pathogens. 2023;12(4):543. 10.3390/pathogens12040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GA AR. A study of clinical features, pleural fluid characteristics, and etiology of exudative pleural effusion in adults. Rajiv Gandhi University of Health Sciences (India); 2006.
- 7.Schwenk HT, Khan A, Kohlman K, Bertaina A, Cho S, Montoya JG, Contopoulos-Ioannidis DG. Toxoplasmosis in pediatric hematopoietic stem cell transplantation patients. Transplantation Cell Therapy. 2021;27(4):292–300. [DOI] [PubMed] [Google Scholar]
- 8.Yusefi M, Arab-Mazar Z, Fallahi S, Mamaghani AJ, Sali S, Nikpour N, Barati M, Rouzbahani AK, Kheirandish F. Diagnosis of Toxoplasma Infection in allogenic pre HCTSP patients using molecular methods. Iran J Parasitol. 2022;17(2):231–9. 10.18502/ijpa.v17i2.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aerts R, Mercier T, Beckers M, Schoemans H, Lagrou K, Maertens J. Toxoplasmosis after allogeneic haematopoietic cell transplantation: experience using a PCR-guided pre-emptive approach. Clin Microbiol Infect. 2022;28(3):440–5. 10.1016/j.cmi.2021.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Wang ZD, Huang SY, Zhu XQ. Diagnosis of toxoplasmosis and typing of Toxoplasma Gondii. Parasit Vectors. 2015;28:8:292. 10.1186/s13071-015-0902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z, Weng X, Xu C, Lin Y, Cheng C, Wei H, Chen W. Metagenomic next-generation sequencing as a diagnostic tool for toxoplasmic encephalitis. Ann Clin Microbiol Antimicrob. 2018;17(1):45. 10.1186/s12941-018-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauwolf KK, Floeth M, Kerl K, Schaumburg F, Groll AH. Toxoplasmosis after allogeneic haematopoietic cell transplantation—disease burden and approaches to diagnosis, prevention and management in adults and children. Clin Microbiol Infect. 2021;27(3):378–88. 10.1016/j.cmi.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Krenzien F, ElKhal A, Quante M, Biefer HRC, Hirofumi U, Gabardi S, Tullius SG. A rationale for age-adapted immunosuppression in organ transplantation. Transplantation. 2015;99(11):2258–68. 10.1097/TP.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCabe R, Chirurgi V. Issues in toxoplasmosis. Infect Dis Clin N Am. 1993;7(3):587–604. PMID: 8254161. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used may be made available by the corresponding author upon reasonable request.