FIG. 5.
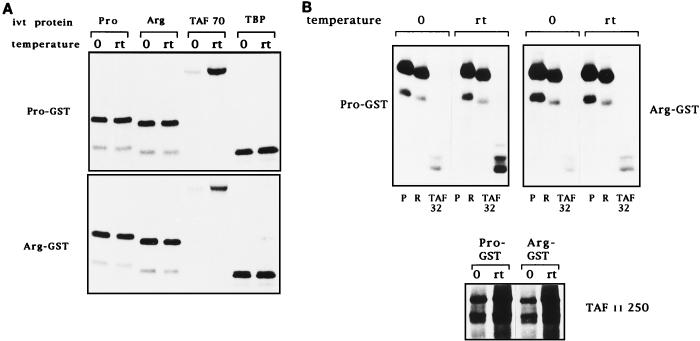
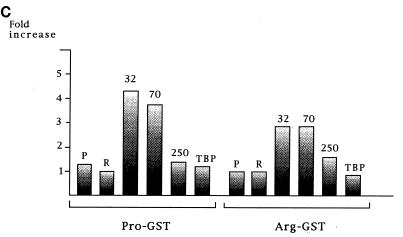
Interactions of p53Pro and p53Arg with transcription factors. (A) GST fusion protein pull-down assay with GST-p53Pro and GST-p53Arg bound to glutathione-agarose. In vitro-translated (ivt) 35S-labeled p53Pro, p53Arg, TAFII70, or TBP proteins were incubated with the fusion protein-agarose at either 0°C or room temperature (rt). The bound proteins were analyzed by SDS-PAGE and autoradiography. No differences in the abilities of p53Pro and p53Arg to form either hetero- or homodimers or in their abilities to bind to TBP were apparent. However, TAFII70 is bound significantly only at room temperature, and p53Pro binds more strongly than p53Arg to TAFII70. (B) The GST-fusion protein pull-down assay repeated with in vitro-translated 35S-labeled TAFII32 and TAFII250 plus p53Pro (P) and p53Arg (R) for comparison. TAFII32 binds more strongly at room temperature and binds more strongly to p53Pro than to p53Arg. TAFII250 binds to p53Pro and p53Arg with similar affinities but binds more strongly at room temperature. (C) Phosphorimager analysis of at least five GST fusion protein pull-down assays. The fold increase in binding at room temperature over binding at 0°C is shown. p53Pro binds approximately twice as strongly as p53Arg to TAFII32 and TAFII70. GST alone was also used as a negative control; in all cases, the percentage of loaded protein retained was less than 1%.