Abstract
Background
To explore the clinical and chest CT features of Talaromycosis marneffei (TSM), and to compare the differences between anti‑IFN‑γ autoantibodies‑positive and HIV-positive cases.
Methods
Clinical data and chest CT images of 54 HIV-negative patients with Talaromyces marneffei (TM) infection and positive anti-interferon-γ (anti-IFN-γ) autoantibody were retrospectively analyzed. Ninety-three HIV-positive patients with TM infection during the same period were included as controls. Differences between groups were compared by the two-sample t-test and the Mann-Whitney U test, respectively.
Results
The time from symptom onset to diagnosis of TSM with positive anti-IFN-γ autoantibodies was significantly longer than that of controls (P < 0.001). BALF/sputum and lymph nodes were the better specimens for detecting TM in the anti-IFN-γ autoantibody-positive group, while TM were more often isolated or detected in blood samples in the other group. Patients with TM infection tended to present with cough, expectoration, and fever. Compared with controls, anti-IFN-γ antibody-positive patients with TM infection had a higher incidence of shortness of breath, skin lesions, joint pain, and high CD4/CD8 ratio (P < 0.05). There were only 1 and 6 cases with negative lung involvements in CT scans in anti-IFN-γ autoantibody-positive patients and HIV-positive patients with TM infection, respectively. In the remaining patients, chest CT manifestations were diverse, mainly presenting as fibrous cord‑like lesions, lymph node enlargement, and sporadic nodules. The incidences of patchy consolidation, air bronchogram, pleural effusion and pleural thickening were higher in anti-IFN-γ autoantibody-positive patients with TM infection than those of controls (P < 0.05).
Conclusions
Anti-IFN-γ antibody positive patients with TM infection showed a longer diagnostic interval. clinical specimens from which TM was isolated or detected were different between the two groups. The clinical and imaging manifestations of patients with TM infection are characteristic.
Keywords: Talaromyces marneffei infection, Anti-IFN-γ autoantibodies, HIV, Computed tomography, Clinical laboratory tests
Introduction
Talaromycosis marneffei (TSM) is an invasive fungal disease that can cause severe systemic infection. It is prevalent in southeast Asia, especially Hong Kong, Thailand, Vietnam, Taiwan, Southern China, Laos, Cambodia, Malaysia and Myanmar. With the increase in the floating population, the epidemic of TSM has far exceeded the original epidemic areas. Approximately 17,300 cases of TSM and 4900 related deaths occur annually [1]. Talaromyces marneffei (TM) is an important intracellular fungal pathogen. After entering the human body through the respiratory tract, digestive tract and damaged skin, TM can replicate in macrophages in the form of yeast, causing local infections in the skin and lungs, and even severe disseminated infection. Historically, TM infection used to be considered occur in people with positive HIV [2]. In recent years, improvements in the treatment of HIV infection have changed the epidemiology of TM infection, and the number of HIV-negative patients with TM infection is on the rise [3]. The anti-IFN-γ autoantibody is able to neutralize IFN-γ, which can severely weaken the scavenging effect of macrophages on phagocytic pathogens, leading to lethal diseases via immune escape and bloodstream spread [4]. Immunodeficiency caused by anti-interferon-γ (anti-IFN-γ) autoantibodies is an adult-onset immunodeficiency syndrome. Positive anti-IFN-γ autoantibody is linked with TM-induced severe or persistent infection in HIV-negative patients, which is one of the high-risk factors [5]. TM infections can affect the lymph nodes, liver, spleen, bone marrow, skin, respiratory system and nervous system, with lung involvements being the most common. It is reported that clinical symptoms of TM infection and lung involvements differ a lot in HIV-positive and HIV-negative patients [6]. TM infection with lung involvements presents similar symptoms as bronchitis, bronchiectasis, acute respiratory distress syndrome, or tuberculosis, which makes the diagnosis complicated [7]. Chest CT can visualize lung involvement after TM infection, showing an important diagnostic value. Therefore, it is of some significance to summarize the characteristics of chest CT after TM infection in different populations. The main chest CT findings of HIV-positive patients with TM infection included ground-glass opacities, consolidation, mediastinal or hilar lymphadenopathy, which were mostly bilaterally distributed [8]. So far, clinical and chest CT manifestations of HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody have been rarely reported. This study aims to retrospectively analyze clinical and chest CT manifestations of HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody in the First Affiliated Hospital of Guangxi Medical University, thus providing references for better clinical decision-making and treatment.
Methods
Clinical laboratory tests
Clinical laboratory tests of 147 TM infection cases diagnosed in the First Affiliated Hospital of Guangxi Medical University from December 2012 to September 2019 were retrospectively analyzed, involving 54 negative-HIV patients with positive anti-INF-γ autoantibody, and 93 positive-HIV patients.
CT data
Conventional chest CT scanning was performed in every subject using the GE 64 Scanner (United States) and Siemens dual-source CT. Briefly, chest CT scanning from the tip to the bottom of the lungs was performed at the end of deep inspiration, with 120 kV tube voltage, 150 mA tube current, 1 mm layer thickness and distance of reconstructed images. The scanned images were observed on the lung window and the mediastinal window. Two experienced radiologists with 26 and 5 years of experience independently analyzed the CT manifestations, lesion distribution and CT scores, and any disagreement was dissolved by discussing with the third senior radiologist in the cardiothoracic group.
Analyses of clinical and imaging manifestations
Clinical and chest CT manifestations of 147 patients with TM infection were analyzed, and CT scores of lung involvements were graded.
Clinical manifestations included respiratory system manifestations (e.g., cough, expectoration, chest tightness, chest pain, shortness of breath) and extrapulmonary manifestations (e.g., fever, skin lesions, abdominal pain, diarrhea, joint pain). Laboratory tests included CD4/CD8 ratio, white blood cell count, lymphocyte count, neutrophil count, platelet count and hemoglobin level.
Chest CT scans were mainly analyzed for lung manifestations, bronchial changes, pleural changes and extrapulmonary manifestations. Lung manifestations were classified into the following 9 subcategories: ground-glass opacity (GGO), patchy consolidation, fibrous cord‑like lesions, small nodules (< 5 mm), nodules (> 5 mm, <3 cm), solid masses (> 3 cm), vacuole sign, emphysema, and interlobular septum thickening. Bronchial changes included air bronchogram, and thickening of the bronchial vascular bundle. Pleural changes included pleural thickening and effusion. Extrapulmonary manifestations included pericardial effusion, and enlarged hilar/mediastinum/supraclavicular fossa lymph nodes.
CT scores were graded by firstly dividing lungs into the upper area (above the carina), the middle area (from the carina to the inferior pulmonary vein) and the lower area (below the inferior pulmonary vein), which were further subdivided into the anterior zone (the zone before the vertical line at the midpoint of the diaphragm in the sagittal position) and the posterior zone (the zone after the vertical line of the midpoint of the diaphragm in the sagittal position), with a total of 12 areas in both lungs. CT scores of lung involvement in each area were graded as follows: 0, no involvement; 1, < 25% involvement; 2, 25–50% involvement; 3, 50–75% involvement; 4, ≥ 75% involvement. CT scores in each area was recorded, with the maximum at 48 points [9]. The distribution of lung lesions was classified as central or peripheral areas.
Statistical analysis
Statistical analysis was performed using SPSS 22.0. Normally distributed measurement data were expressed as  ±s; Otherwise, they were expressed as M (Q1, Q3). Differences between groups were compared by the two-sample t-test and the Mann-Whitney U test, respectively. Enumeration data were expressed as n and percentage (%), and differences between groups were compared by the Chi-square test. P < 0.05 considered as statistically significant.
±s; Otherwise, they were expressed as M (Q1, Q3). Differences between groups were compared by the two-sample t-test and the Mann-Whitney U test, respectively. Enumeration data were expressed as n and percentage (%), and differences between groups were compared by the Chi-square test. P < 0.05 considered as statistically significant.
Results
Clinical laboratory tests
Among 54 anti–IFN-γ autoantibodies-positive patients and 93 HIV-positive patients, they were diagnosed as TSM based on blood culture (n = 79, 8% vs. 92%), bone marrow culture (n = 19, 37% vs. 63%), bronchoalveolar lavage fluid (BALF)/sputum culture (n = 17, 88% vs. 12%), fungal culture and identification via lymph node biopsy (n = 11, 91% vs. 9%), fungal culture and identification of skin purulent secretions (n = 10, 90% vs. 10%), skin lesion biopsy and fungal culture (n = 7, 57% vs. 43%), and fungal culture via pulmonary biopsy (n = 4, 75% vs. 25%). Some patients have been tested for multiple diagnostic methods, and only one of them was shown above. Anti–IFN-γ autoantibodies were detected (OD > 0.5, in 1:100 dilution) in these 54 patients. BALF/sputum and lymph nodes were the better specimens for detecting TM in the anti-IFN γ autoantibody-positive group, while TM were more often isolated or detected in blood samples in the other group.
The clinical manifestations of TSM are diverse (Table 1). The time from symptom onset to diagnosis of TSM with positive anti–IFN-γ autoantibodies (n = 54, median: 150.5 days, range: 29-1213 days) was significantly longer than that of controls (n = 93, median: 35.8 days, range: 18–362 days, P < 0.001). Patients with TM infection tended to present with cough, expectoration, and fever. Compared with controls, anti-IFN-γ antibody-positive patcnd high CD4/CD8 ratio (P < 0.05). Twenty-seven of the 54 patients were initially misdiagnosed as tuberculosis. Skin lesions were present in 19 of 54 anti-IFN-γ autoantibody positive TSM patients, of which 5 cases were Sweet’s syndrome (Figs. 1 and 2 [10]). CD4/CD8 ratio, white blood cell count, lymphocyte count, neutrophil count, and platelet count were usually elevated or normal in anti-IFN-γ autoantibody- positive patients with TM infection, which were mainly reduced or normal in controls (P < 0.05).
Table 1.
Clinical manifestations of patients with TM infection (n = 147)
| Characteristic | anti-IFN-γ autoantibody(+)(n = 54) | HIV (+) (n = 93) | Statistic | P |
|---|---|---|---|---|
| Gender | 10.699a | 0.001 | ||
| Male | 33 (61%) | 79 (85%) | ||
| Female | 21 (39%) | 14 (15%) | ||
| Age (years) | 53. 0 ± 11.3 | 39.8 ± 12.8 | 6.288c | <0.001 |
| Diagnostic (days) | 150.5 (81.0, 288.5) | 35.8 (27.3, 92.6) | 960.0b | <0.001 |
| Clinical manifestations | ||||
| Fever | 46 (85%) | 75 (81%) | 0.484a | 0.487 |
| Cough/Expectoration | 43 (80%) | 47 (51%) | 12.179 a | <0.001 |
| Chest tightness/pain | 11 (20%) | 6 (6%) | 6.471 a | 0.011 |
| Shortness of breath | 8 (15%) | 3 (3%) | 5.059 a | 0.024 |
| Skin lesions | 28 (51%) | 30 (32%) | 5.491 a | 0.019 |
| Abdominal pain/Diarrhea | 10 (19%) | 28 (30%) | 2.394 a | 0.122 |
| Joint pain | 19 (35%) | 2 (2%) | 30.447 a | <0.001 |
| Laboratory tests | ||||
| CD4/CD8 | 1.2(0.8,1.4) | 0.1(0.0, 0.2) | 60.5b | <0.001 |
| White blood cell count (×109/L) | 11.5(8.6, 15.2) | 3.7(2.7, 4.9) | 459.5 b | <0.001 |
| Lymphocyte count (×109/L) | 1.0(2.0, 3.0) | 0.4(0.2, 0.7) | 562.5 b | <0.001 |
| Neutrophil count (×109/L) | 8.4(5.09, 11.00) | 2.8(1.8, 3.9) | 645.5 b | <0.001 |
| Hemoglobin level (g/dL) | 9.01 ± 1.98 | 9.30 ± 9.29 | 1.010c | 0.32 |
| Platelet count (×109/L) | 334.0(248.0, 451.1) | 129.7(80.4, 222.9) | 750.5 b | <0.001 |
Note— a = Chi-square test, b = Mann-Whitney U test, c = t-test
Fig. 1.
Rash manifestations of Sweet syndrome in Talaromycosis marneffei with positive anti‑IFN‑γ autoantibodies. The picture is excerpted from the research of Fu et al. [9]. It has been approved by the author. (A) Red round plaques with clear boundaries in the hand, with pseudovesicular changes. (B) Edematous purplish red plaques in the hand. (C) Purplish red round plaques in the hand
Fig. 2.
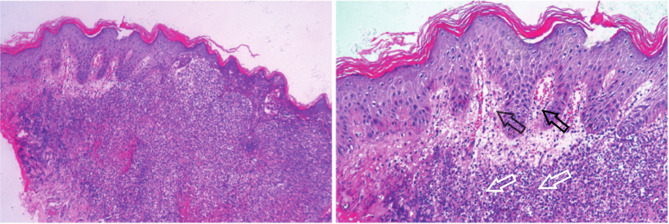
Hand Sweet syndrome rash histopathology Dermal papilledema, diffuse neutrophil-dominated inflammatory cell infiltration in the dermis (HE × 50, × 100). The black arrows indicate dermal papilledema. The white arrows indicate inflammatory cell infiltration
Each patient was treated with 1 or 2 antifungals, including voriconazole, itraconazole, fluconazole or amphotericin B, and returned to the hospital for re-examination of chest CT every one to three months after the first antibacterial treatment. Of the 54 patients, 43 were followed up for a period of 7–63 months, with a median of 22.0 (14.0-31.5) months. The outcomes of these 43 patients were as follows: 13 patients died, 19 patients had recurrent TM infections or developed other opportunistic infections, and 8 patients were cured (cure defined as sustained remission 6 months after the end of treatment).
Chest CT manifestations
Most patients with TM infection had abnormal chest CT findings. There were 1 and 6 cases with negative lung involvements in CT scans in anti-IFN-γ autoantibody-positive patients and HIV-positive patients with TM infection, respectively. The more frequent chest CT signs of 54 anti-IFN-γ autoantibody positive patients with TM infection were fibrous cord‑like lesions (87%, 47/54), enlarged lymph nodes (76%, 41/54), sporadic nodules (> 5 mm, <3 cm) (74%, 40/54), consolidation (72%, 39/54), air bronchogram (63%, 34/54), pleural effusion (59%, 32/54) and pleural thickening (59%, 32/54). The incidences of the above-mentioned CT manifestations were significantly higher than those of HIV-positive patients with TM infection (all P < 0.05). Chest CT lesions in two groups were mainly bilaterally distributed, and most of them showed both peripheral and central involvements, with 2 or more affected lung lobes. The rest of chest CT manifestations and distribution were shown in Table 2; Fig. 3.
Table 2.
Chest CT manifestations of patients with TM infection (n = 147)
| CT Feature | anti-IFN-γ autoantibody (+) (n = 54) | HIV (+) (n = 93) | X2 | P |
|---|---|---|---|---|
| Lung change | ||||
| Consolidation | 39 (72%) | 22 (24%) | 33.192 | <0.001 |
| GGO | 24 (44%) | 26 (28%) | 4.138 | 0.042 |
| Fibrous cord‑like lesions | 47 (87%) | 50 (54%) | 16.582 | <0.001 |
| Sporadic nodules(>5 mm, <3 cm) | 40 (74%) | 52 (56%) | 4.811 | 0.028 |
| Diffuse nodules(<5 mm) | 6 (11%) | 16 (17%) | 0.997 | 0.318 |
| Solid masses(>3 cm) | 11 (20%) | 11 (12%) | 1.959 | 0.162 |
| Vacuole sign | 15 (28%) | 21 (23%) | 0.499 | 0.480 |
| Emphysema | 16 (30%) | 23 (25%) | 0.421 | 0.517 |
| Interlobular septum thickening | 12 (22%) | 7 (8%) | 6.555 | 0.010 |
| Bronchial change | ||||
| Air bronchogram | 34 (63%) | 11 (12%) | 42.055 | <0.001 |
| Thickening of the bronchial vascular bundle | 9 (17%) | 9 (10%) | 1.553 | 0.213 |
| Pleural change | ||||
| Pleural effusion | 32 (59%) | 23 (25%) | 17.394 | <0.001 |
| Thickening of pleura | 32 (59%) | 9 (10%) | 41.759 | <0.001 |
| Extrapulmonary manifestations | ||||
| Pericardial effusion | 10 (19%) | 17 (18%) | 0.001 | 0.971 |
| Enlarged lymph nodes | 41 (76%) | 39 (42%) | 15.913 | <0.001 |
| Lesion distribution | ||||
| Bilateral | 42 (78%) | 68 (73%) | 0.394 | 0.530 |
| Peripheral and central | 38 (70%) | 46 (49%) | 6.098 | 0.014 |
| ≥2 lobes affected | 49 (90%) | 73 (78%) | 3.630 | 0.057 |
Note—GGO = ground-glass opacities
Fig. 3.
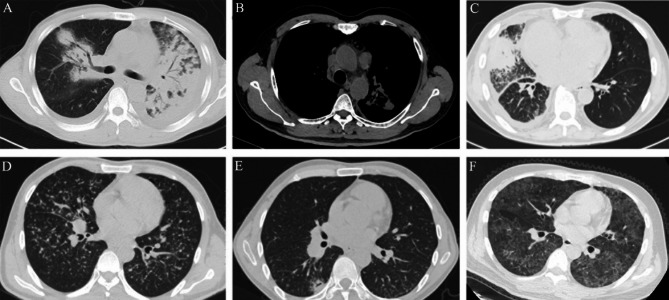
Chest CT findings of Talaromycosis marneffei. (A) A 46-year-old male anti‑IFN‑γ autoantibodies‑positive patient showing a large consolidation of the left lung, a mass in the right lung, air bronchogram and bilateral pleural effusion in the chest CT scan. (B) A 58-year-old male anti‑IFN‑γ autoantibodies‑positive patient showing enlarged mediastinal lymph nodes in the chest CT scan. (C) A 63-year-old male anti‑IFN‑γ autoantibodies‑positive patient showing a mass in the middle lobe of the right lung and pleural effusion in the chest CT scan. (D) A 31-year-old HIV-positive patient with TM infection showing diffuse miliary nodules in both lungs in the chest CT scan. (E) A 35-year-old HIV-positive patient with TM infection showing a nodule in the lower lobe of the right lung with small vacuoles. (F) A 37-year-old HIV-positive patient with TM infection showing diffuse ground glass foci in both lungs in the chest CT scan
The degree of lung involvements of 147 patients with TM infection varied a lot, with CT scores ranging 0–48 points. The median CT scores and the interquartile range in anti-IFN-γ autoantibody-positive patients were 11.5 (6, 19) points, which were 6.0 (2, 27) points in HIV-positive patients. No significant difference in CT scores of lung involvements was detected between groups (U = 2133.5, P = 0.129).
Discussion
In 2004, Germany scientists [11, 12] proposed that acquired adult immunodeficiency syndrome caused by anti-IFN-γautoantibodies is an emerging adult-onset immunodeficiency disease. Anti-IFN-γ autoantibody-mediated immunodeficiency syndrome often manifests as Sweet’s syndrome or severe systemic infection. However, the specific mechanism of why Sweet syndrome occurs in anti-IFN-γ autoantibody-positive TSM remains to be studied. Nontuberculous mycobacteria and TM are mainly responsible for opportunistic infections [13]. Yang et al. [14] showed that the TM-derived extracellular vesicles could mediate inflammatory response and its protein would play a key role in regulating the function of RAW 264.7 macrophage cells. The present study show that the condition following TM infection varies among patients or even within the same patient over time. This result fits with Damage Response Framework’s view that: (I) microbial pathogenesis results from the interaction between a host and a microbe; (II) host damage can arise from microbial (fungal) factors, host factors, or a combination of both [15]. Consistent with a previous report in Southeast Asian cases [16], TM was more likely to be detected or isolated in blood specimens in HIV-positive cases. In the present study, clinical specimens from which TM was isolated or detected were different between the two groups. In the anti-IFN-γautoantibody-positive group, TM was more commonly isolated or detected in BALF/sputum(27.8%) and lymph node (18.5%), while in the HIV-positive group, the blood specimens was better (78.5%). This situation is probably related to the fact that TM causes a different clinical spectrum in the two patient groups and thus TM is isolated from a different clinical sample. Anti-IFN-γ antibody positive patients with TM infection showed a longer diagnostic interval. This may be because TM infection progresses more rapidly in HIV-positive patients, and these cases often present with fever with specific skin rashes, and most of the pathogens can be isolated from blood specimens, making diagnosis easier. However, in the anti-IFN-γ antibody positive group, the presence site of TM is hidden, the specimen is difficult to collect, and the positive rate of culture is low, which can easily lead to misdiagnosis. Clinical manifestations of TM infection are diverse, which are similar to those of other opportunistic infectious diseases that are difficult to be differentiated and diagnosed. Previous evidences have shown that symptoms and signs of TM infection are atypical, including fever, weight loss, fatigue, hepatosplenomegaly, lymphadenopathy, respiratory and gastrointestinal abnormalities [6]. Skin lesions can be the single or first symptom of TM infection [2, 17]. In the present study, fever, cough and expectoration were the most-common symptoms of anti-IFN-γ autoantibody-positive patients with TM infection, and some of them presented abdominal symptoms and skin lesions, which were consistent with previous findings. Compared with HIV-positive TSM cases, the incidences of shortness of breath and joint pain in anti-IFN-γ autoantibody positive TSM cases were significantly higher, while those of fever, skin lesions, abdominal pain/diarrhea were significantly lower (all P < 0.05). Inconsistently, Li et al. [18] reported that there are no significant differences in the incidences of fever, cough and abdominal pain between HIV-positive and HIV-negative patients with TM infection. In this study, the symptoms were not identical in the two groups, with respiratory and joint symptoms more common in the anti-IFN-γ autoantibody-positive group. On the one hand, human TM acquisition occurs after inhalation of conidia or hyphal fragments and their deposition in the lungs. Severe systemic disseminated infections often occur in cases of adult-onset immunodeficiency syndrome induced by anti-IFN-γ autoantibody, in which lungs are one of the most affected organs [19]. On the other hand, these patients are more prone to reversible bone destruction, resulting in a greater probability of bone and joint pain. CD4/CD8 ratio was significantly decreased in HIV positive patients, which was consistent with previous studies. HIV positive patients with low CD4 cell count had a significantly increased risk of TM infection [20].
Chest CT manifestations of HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody were diverse. Fibrous cord‑like lesions were the most-common CT manifestations in HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody, the incidence of which was significantly higher than that of HIV-positive patients with TM infection (P < 0.05), which may be attributed to the disease duration. The duration from symptom onset to diagnosis of HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody was significantly longer than that of controls (P < 0.001), suggesting that the disease course was longer in the former. In anti-IFN-γ autoantibody-positive patients with TM infection, the absorption and fibrosis of some local lung lesions may have occurred prior to the first chest CT examination due to its long diagnostic interval. Lung involvements of patients with TM infection have a complicated pattern involving both lung parenchyma and lung interstitium in CT scans. Here, consolidation was more commonly detected in chest CT scans of HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody, while interlobular septal thickening was less observed. It is suggested that lung parenchyma was mainly affected by TM infection. Yuan et al. [21] reported that the incidences of lymphadenopathy and lung nodules are relatively higher in HIV-positive TM patients, while those of pleural effusion and lung consolidation are low, which were consistent with our findings. In addition, the incidences of pleural effusion and thickening were higher in HIV-negative patients with TM infection and positive anti-IFN-γ autoantibody than those of HIV-positive patients with TM infection, suggesting that the pleura was more commonly affected in the latter population. Vacuole sign is induced by the incomplete filling of alveolar clusters in the exudative lung parenchyma [9]. The appearance of the vacuole sign or vacuoles indicates the possibility of fungal infection, which is conductive to the clinical diagnosis. Chest CT results showed that the CT scores of lung involvements of two groups were similar. The distribution of chest CT lesions in two groups were mainly bilateral, and most of them showed both peripheral and central involvement, with more than 2 lung lobes involved in. We considered that TM infection is disseminated caused by the impaired immune system, and lungs were extensively affected, leading to evenly distributed and diffuse lesions. In cases of anti-IFN-γ autoantibody-positive TSM, some patients presented osteolytic bone destruction of the scapula, sternum, or ribs on chest CT scans, but these characteristic signs are rare in HIV-positive patients. It is reported that osteolytic destruction is reversible in patients with TM infection, presenting a repeated and migratory bone destruction-bone repair process during follow-up, which can be used to differentiate from bone destruction caused by malignant tumors [22].
This study had the following limitations: (1) Sample size was small; (2) CT manifestation changes during the whole course of disease were failed to be tracked and analyzed; (3) The correlation between the titer of anti-IFN-γ autoantibody and the degree of lung involvements was failed to be assessed; (4) The study has only collected data from 2012 to 2019, and data from 2020 onwards are still in a pre-processing state. In the future it will be necessary to update the data of recent years. The focus is on long-term follow-up to assess the diagnosis and treatment, thereby increasing the level of awareness of TSM.
Conclusion
Anti-IFN-γ antibody positive patients with TM infection showed a longer diagnostic interval. clinical specimens from which TM was isolated or detected were different between the two groups. In the anti-IFN-γautoantibody-positive group, TM was more commonly isolated or detected in BALF/sputum and lymph node. The clinical and imaging manifestations of anti-IFN-γ antibody-positive patients with TM infection were characterized. Analysis of chest CT characteristics combined with clinical data contributes to enhance the diagnostic accuracy.
Acknowledgements
Thanks to Professor Cao CW and Professor Fu YJ for their support in data collection and case figure. This study was supported by the First Affiliated Hospital of Guangxi Medical University and the First Affiliated Hospital of Guangxi University of Chinese Medicine.
Abbreviations
- TSM
Talaromycosis marneffei
- TM
Talaromyces marneffei
- anti-IFN-γ
Anti–interferon-γ
- AIDS
Acquired immunodeficiency syndrome
Author contributions
WXT and ZYH wrote the main manuscript text. WXT, ZYH, and WCC collected clinical data. LYM, LZF, and LK conceived the study and revised the manuscript. WXT and ZYH ‘s contributions to this study were consistent.
Funding
Not applicable.
Data availability
Data relating to this study are contained and presented in this document. Other materials are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki, and approved by the First Affiliated Hospital of Guangxi Medical University in Nanning City, Guangxi, China (No. 2021KY-E-233). The need for informed consent was waived because this study was retrospective.
Consent for publication
Informed written consent for publication including all the photos was obtained from the patient by the corresponding author and available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yumin Lu, Email: luym2008@sina.com.
Kai Li, Email: doctorlikai@126.com.
References
- 1.Narayanasamy S, Dat VQ, Thanh NT, Ly VT, Chan JF, Yuen KY, et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Global Health. 2021;9:e1618–22. 10.1016/s2214-109x(21)00350-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu YX, Zhang JM, Li XQ, Yang YB, Zhang Y, Ma JH, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175:57–67. 10.1007/s11046-012-9577-0 [DOI] [PubMed] [Google Scholar]
- 3.Zeng W, Qiu Y, Tang SD, Zhang JQ, Pan ML, Zhong XN. Talaromyces marneffei Characterization of Anti-Interfeantibodiesbodies in HIV-Negpatientstinfectedfwithddisseminatedinated and Cryptococcosis. Open Forum Infect Dis. 2019;6:ofz208. 10.1093/ofid/ofz208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725–34. 10.1056/NEJMoa1111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi NN, Kong JL, Wang K, Cao CW. Coinfection with Talaromyces marneffei and other Pathogens Associated with Acquired Immunodeficiency. JAMA Dermatol. 2019;155:1195–7. 10.1001/jamadermatol.2019.1532 [DOI] [PubMed] [Google Scholar]
- 6.Cao CW, Xi LY, Chaturvedi V, editors. (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia. 2019;184:709–720. 10.1007/s11046-019-00410-2 [DOI] [PubMed]
- 7.De Monte A, Risso K, Normand AC, Boyer G, L’Ollivier C, Marty P, et al. Chronic pulmonary penicilliosis due to Penicillium marneffei: late presentation in a French traveler. J Travel Med. 2014;21:292–4. 10.1111/jtm.12125 [DOI] [PubMed] [Google Scholar]
- 8.Shi XD, Huang SW, Zan Y, He XY, Li Y, Peng DC, Liu JG, Zhong Z, Yu QZ. Chest CT findings of HIV-related penicillium marneffei infection. Radiol Practice. Chest CT manifestation of AIDS patients complicated with Penicilliosis marneffei Hainan Med J. 2016:2474–2475. https://doi.org/doi:10.3969/j.issn.1003-6350.2016.15.021.
- 9.Zhou SC, Wang YJ, Zhu TT, Xia LM. CT features of Coronavirus Disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;1–8. 10.2214/AJR.20.22975 [DOI] [PubMed]
- 10.Fu YJ, Guo J, Shi NN, Qiang NX, Wei FL, Zheng YQ, et al. CT features of Coronavirus Disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;1–8. 10.2214/AJR.20.22975 [DOI] [PubMed]
- 11.Höflich C, Sabat R, Rosseau S. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103(2). 10.1182/blood-2003-04-1065 [DOI] [PubMed]
- 12.Döffinger R, Helbert MR, Barcenas-Morales G. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38:1. https://doi.10.1086/380453. [DOI] [PubMed]
- 13.Sisto F, Miluzio A, Leopardi O, Mirra M, Boelaert JR, Taramelli D. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infection. 2003;71:465–73. 10.1128/iai.71.1.465-473.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Wang JY, Jiang HY, Lin HX, Ou ZH, Ullah A. Extracellular Vesicles Derived From Talaromyces marneffei Yeasts Mediate Inflammatory Response in Macrophage Cells by Bioactive Protein Components. Front Microbiol2020;11:0 https://doi.10.3389/fmicb.2020.603183. [DOI] [PMC free article] [PubMed]
- 15.Pruksaphon k, Pruksaphon A, Jeenkeawpieam J, Thammasit P, Nosanchuk JD, Youngchim S. The microbial damage and host response framework: lesson learned from pathogenic survival trajectories and immunoinflammatory responses of Talaromyces marneffei infection. Front Immunol. 2024;15:0.:https://doi.10.3389/fimmu.2024.1448729. [DOI] [PMC free article] [PubMed]
- 16.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344:110–3. 10.1016/s0140-6736(94)91287-4 [DOI] [PubMed] [Google Scholar]
- 17.Chan JF, Lau SK, Yuen KY, Woo PC. infections. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerging microbes. 2016;5:e19. 10.1038/emi.2016.18 [DOI] [PMC free article] [PubMed]
- 18.Li HR, Cai SX, Chen YS, Yu ME, Xu NL, Xie BS, et al. Comparison of Talaromyces marneffei infection in human immunodeficiency virus-positive and human immunodeficiency virus-negative patients from Fujian, China. Chin Med J. 2016;129:1059–65. 10.4103/0366-6999.180520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J, Ning XQ, Ding JY, Zheng YQ, Shi NN, Wu FY, et al. Anti-IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med. 2020;217. 10.1084/jem.20190502 [DOI] [PMC free article] [PubMed]
- 20.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–4. 10.1093/clinids/14.4.871 [DOI] [PubMed] [Google Scholar]
- 21.Yuan MJ, Li SH, Huang Y, Fu WD, Li MY. Clinical imaging analysis and curative effect evaluation of AIDS complicated with Marneffei basket infection. Chin J Infect Dis. 2019;37. 10.3760/cma.j.issn.1000-6680.2019.01.008
- 22.Liang YH, Liu YW, Zeng QS. Chest CT and PET-CT manifestations of talaromycosis marneffei (TSM) in normal immunocompetent patients. Radiol Pract. 2019;34:12. 10.13609/j.cnki.10000313.2019.12.007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data relating to this study are contained and presented in this document. Other materials are available from the corresponding authors on reasonable request.