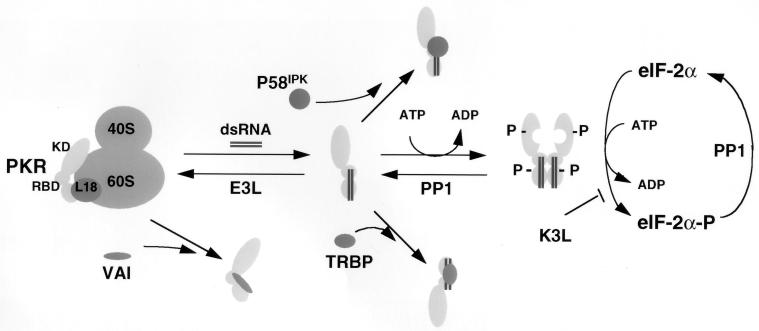
FIG. 7.
PKR inhibitors and their mechanism of action. PKR is depicted as a monomer bound to and inhibited by ribosomal protein L18. Upon accumulation of excess dsRNA, PKR is released from L18 and is susceptible to dsRNA-induced dimerization, autophosphorylation, and activation of the eIF-2α kinase activity. Several viral inhibitors that act through different mechanisms are depicted. Adenovirus VAI RNA binds and inhibits PKR and also displaces it from the ribosome (86). Numerous viral proteins, such as vaccinia virus E3L, bind and sequester dsRNA, thereby reversing the equilibrium from dsRNA activation to L18-mediated inhibition. Vaccinia virus encodes a protein, K3L, that acts as a pseudosubstrate for PKR (77). Protein phosphatase PP1 acts to dephosphorylate phosphorylated eIF-2α as well as phosphorylated PKR. Herpes simplex virus type 1 encodes a protein that facilitates activation of PP1 (26). Finally, two cellular inhibitors, P58IPK and TRBP, prevent activation of PKR by interfering with the activation process through inhibition of PKR dimerization. Other cellular inhibitors of PKR, including Alu RNA (14), La antigen (88), and p67 (87), are not depicted here.