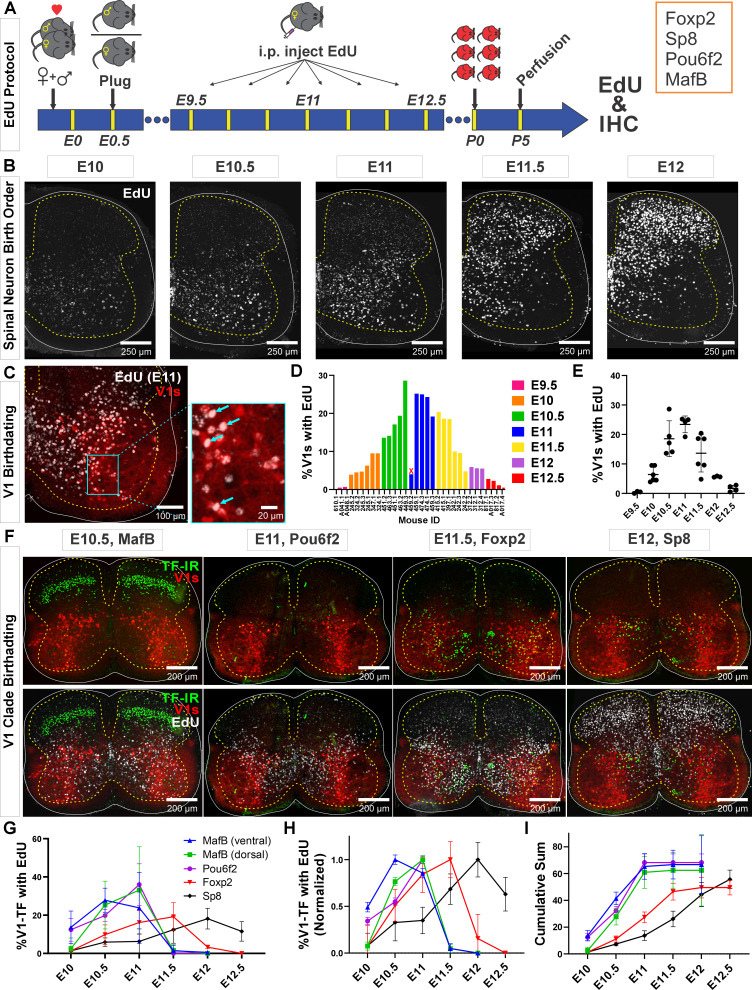
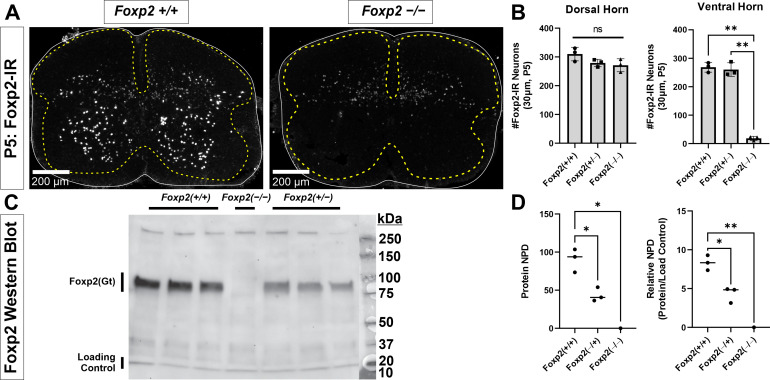
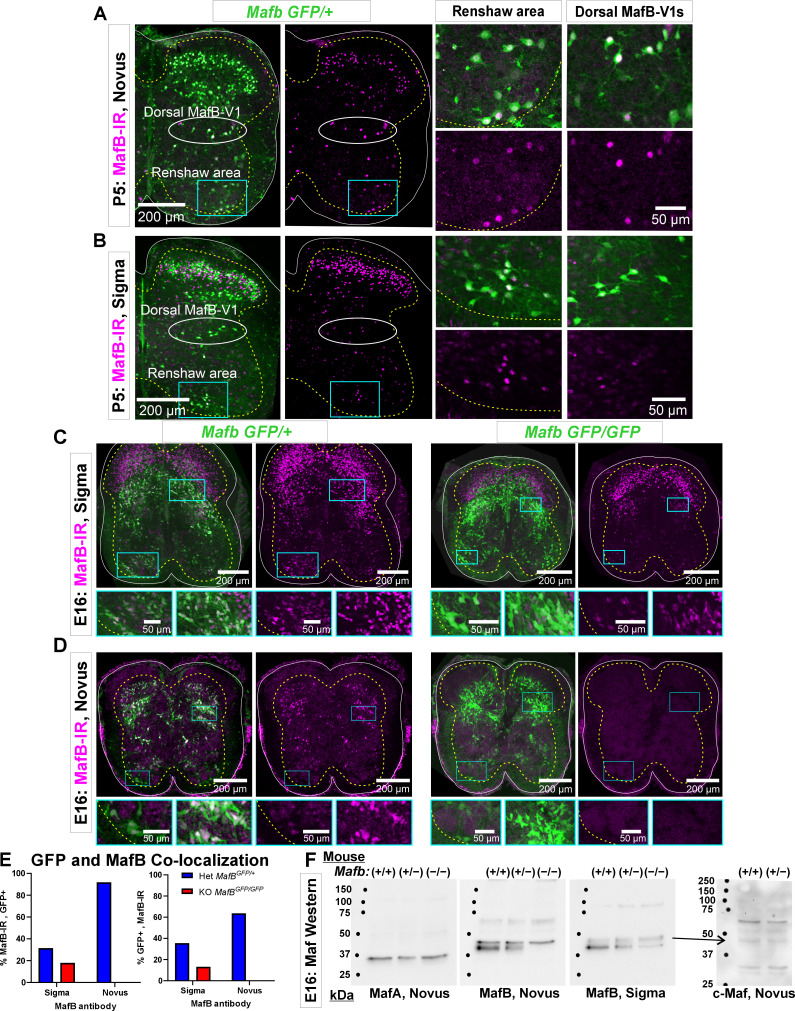
These target sequences are shared with mouse MafA and c-Maf, but the overlap is larger with the Sigma antibody immunogen. A BLAST sequence search found 98%, 53%, and 64% significant alignment between the MafB sequence used for creation of the Sigma antibody with, respectively, aa18–140 in mouse MafB, aa18–143 in mouse MafA, and aa19–118 in mouse c-Maf. The sequence used in the Novus antibody detected no alignment with proteins other than mouse MafB. (
A) P5 spinal cord from a
MafbGFP/+ mouse immunostained with MafB-Novus antibodies. GFP (green) reports
mafb gene expression (see
Figure 1—figure supplement 3). Immunohistochemistry detects MafB protein (Cy3, magenta). Higher magnifications of the indicated areas, including the dorsal and ventral (Renshaw) MafB-V1 groups, are shown to the right. (
B) P5 spinal cord from a
MafbGFP/+ mouse immunostained with MafB-Sigma antibodies. The MafB-Sigma antibody detects many more dorsal horn neurons than the MafB-Novus antibody. Many interneurons at this location are known to express c-Maf. In the ventral horn MafB-immunoreactivity is qualitatively similar for both antibodies. This includes both the regions occupied by V1 Renshaw cells and by dorsal-MafB-V1s (Pou6f2-V1 clade). (
C) Immunoreactivity against MafB-Sigma antibodies in het (
MafbGFP/+) and KO (
MafbGFP/GFP) tissue from E16 MafB-GFP reporter mice (the MafB KO is lethal at P0 because it cannot breathe on its own). Low-magnification images of MafB-GFP and MafB-immunoreactivity combined (left panel) or MafB-immunoreactivity alone (right panel) in the het (left pair) and the KO mouse (right pair). The boxed ventral and dorsal horn areas are magnified below. GFP in MafB-GFP mice reports activity of the
mafb promoter, but the knocked-in GFP inactivates the
Mafb allele. When both alleles carry GFP (KO,
MafbGFP/GFP), GFP reports cells with gene expression from the
Mafb locus although no
Mafb mRNA or protein is produced. In the het mouse one allele produces
Mafb mRNA: in this tissue, there is a high degree of overlap between GFP and protein immunoreactivity in the ventral and deep dorsal horns. However, there is more MafB-Sigma immunoreactivity than GFP in superficial laminae. In the KO, ventral horn immunoreactivity is greatly diminished, but lingering weak immunoreactivity remains in many neurons, including Renshaw cells. This could represent cross-reaction with MafA in the tissue. Most MafB-Sigma immunoreactivity in superficial laminae remains in the KOs suggesting that these cells strongly express a cross-reacting target and frequently do not express
Mafb (GFP negative). This is most likely c-Maf which is highly expressed in laminae I to III neurons. (
D) As in C, for MafB-Novus antibodies. Unlike MafB-Sigma, there is little MafB-immunoreactivity in the het animal outside GFP+ cells reporting MafB expression; this includes superficial laminae cells. All immunoreactivity disappears in the KO animal. (
E) Ventral horn co-localization of MafB-GFP and MafB-immunoreactivity obtained with Sigma and Novus antibodies. Graphs show the percentage of immunoreactive cells that are GFP+ (left) and the percentage of GFP+ cells that co-localize the indicated MafB antibody immunoreactivity (right). In one spinal cord ventral horn section from a het mouse (blue bars), we sampled 441 cells with MafB-Sigma immunoreactivity, but the same section had fewer MafB-GFP cells (n=392) such that only a small number of cells co-localized both markers (n=139). In total, 31.5% of MafB-Sigma immunoreactive cells expressed GFP and 35.5% of GFP+ cells had MafB-Sigma immunoreactivity. Conversely, in a serial section immunolabeled with the Novus antibody we detected fewer MafB-Novus immunoreactive cells (n=149) than MafB-GFP cells (n=215) and the large majority were GFP+ (92.0%, n=137). There was almost no MafB-Novus immunoreactivity outside GFP+ cells. Thus, the MafB-Novus antibody is more restricted to GFP+ cells than the MafB-Sigma antibody. In addition, 63.7% of GFP+ cells express MafB-Novus immunoreactivity and while putative dorsal-MafB V1 cells express strong MafB-Novus immunoreactivity this is weak in Renshaw cells. In one MafB KO section (red bars) we detected 217 MafB-Sigma immunoreactive cells and 296 GFP+ cells with 39 cells co-localizing both markers. This corresponded to 17.9% of MafB-immunoreactive cells expressing GFP and 13.2% of the GFP+ cells expressing MafB-Sigma immunoreactivity. The MafB-Novus antibody showed no MafB-immunoreactivity in KO tissue sections with similar numbers of GFP+ cells (n=241). (
F) Western blots of MafA (Novus), MafB-Novus, and MafB-Sigma on nuclear extracts from one WT (
Mafb+/+),
one Mafb heterozygous (het,
MafbGFP/+), and one
Mafb knockout (KO,
MafbGFP/GFP), all E16 littermates. The same western blot was stripped and re-probed three times with the antibodies in the following order: MafB-Novus, MafA-Novus, and finally MafB-Sigma. The MafA antibody shows a single band just below the 37 kDa marker, aligning with a predicted molecular weight of 37.6 kDa. The band is of similar size in all three lanes suggesting no compensatory change in MafA expression in
Mafb hets and KOs. Neither of the MafB antibodies detected the MafA band in western blots, suggesting that any possible cross-reaction in tissue is due to IgG species detecting secondary or tertiary protein structures. MafB antibodies generated a double band, with the upper band being weaker than the lower band for MafB-Sigma compared to MafB-Novus. The immunoreactivity of the lower band to MafB-Sigma diminished in hets compared to WTs and diminished further in the KO. This band completely disappeared in the KO probed with MafB-Novus antibodies. This suggest that this band corresponds to MafB and occurs at approximately 40 kDa, slightly over the predicted 35.8 kDa molecular weight. Both antibodies detected a higher molecular weight band that does not change with gene dose. This could correspond to c-Maf with a larger predicted molecular weight, 38.5 kDa. Therefore, we performed a new western blot using a c-Maf antibody from Novus, and we found a correspondence between the upper band detected by both MafB antibodies with one of the bands in the c-Maf western blots. This suggests that in western blots both MafB antibodies cross-reacted with c-Maf. Raw images of the blots and corresponding labeling are found in
Figure 1—figure supplement 2—source data 1,
Figure 1—figure supplement 2—source data 2, and
Figure 1—figure supplement 2—source data 3.
Characterization of MafB (Novus, NB600-266) and MafB (Sigma, HPA00563) antibody immunoreactivities in the spinal cord.