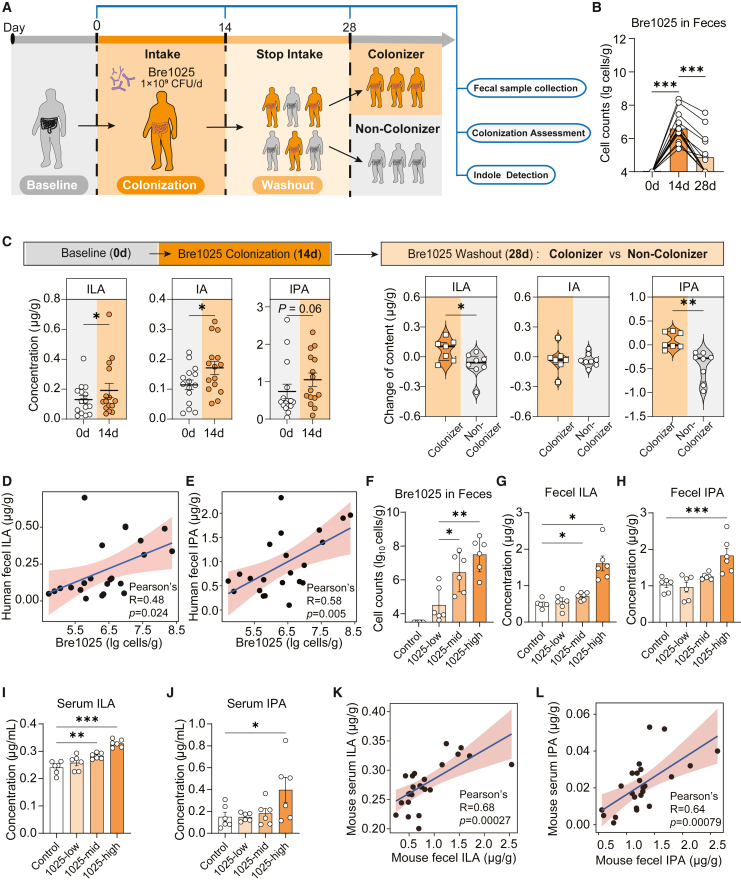
Figure 2.
Ingestion of Bre1025 improved host gut and serum ILA levels
(A) Schematic representation of the clinical trial experimental strategy. In this clinical trial, 15 healthy subjects were recruited for testing the colonization amount and ILA production capability of Bre1025.
(B) Quantification of Bre1025 cell counts in human feces.
(C) Content changes in indole derivatives in human feces.
(D and E) Correlation between Bre1025 biomass and ILA/IPA content in human feces.
(F) Quantification of Bre1025 cell counts in mouse feces. In this animal experiment, 24 mice were randomly divided into four groups for the quantitative analysis of intestinal and serum indole derivatives (n = 6/treatment): control (blank control group), 1025-low (low-dose Bre1025 intervention group, 1 × 10∧7 CFU/d), 1025-med (medium-dose Bre1025 intervention group, 1 × 10∧8 CFU/d), and 1025-high (high-dose Bre1025 intervention group, 1 × 10∧9 CFU/d).
(G and H) Quantification of ILA/IPA concentrations in mouse feces.
(I and J) Quantification of ILA/IPA concentrations in mouse serum.
(K) Correlation between fecal ILA content and serum ILA content in mouse feces.
(L) Correlation between fecal IPA content and serum IPA content in mouse feces.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, determined by two-tailed paired Student’s t test in (B) and (C) and one-way ANOVA followed by Sidak post hoc test in (F–J).