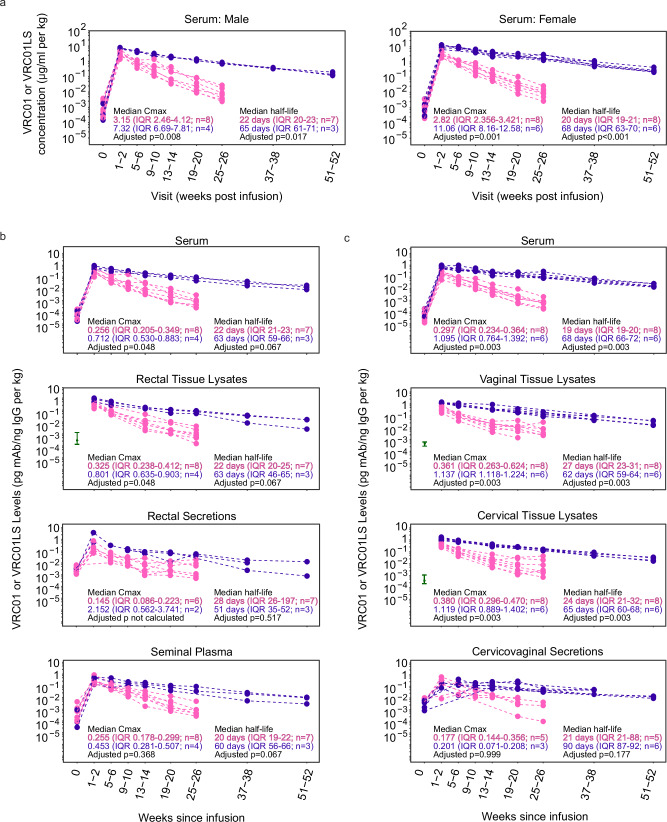
Fig. 2. VRC01LS infusion leads to a higher mucosal Cmax and longer mucosal half-life compared to VRC01 infusion.
a Serum mAb concentrations were measured using Singulex Erenna single-molecule counting technology in participants who received a single IV infusion of 30 mg/kg VRC01 (pink) or VRC01LS (violet). Serum samples were diluted at 1:1000 to 1:50,000 for quantitation using 5C9 in the bead-based assay. Pre-infusion (timepoint 0) serum concentrations are included for reference and were comparable among both mAb groups (p = 0.852 for females, p = 0.562 for males). b IgG-normalized concentrations of VRC01 (pink) and VRC01LS (violet) in males receiving VRC01 or VRC01LS. Serum, rectal biopsy lysates, rectal secretions, and semen are depicted in individual graphs. Rectal biopsies were not collected pre-infusion (timepoint 0), so the baseline comparator (green interval line) is the 25–75 percentile range of pre-infusion samples collected from the other HVTN 116 study arms. Fourteen of the rectal secretions (n = 4 males; 21.5% of collections) were excluded due to evidence of high hemoglobin, an evidence of blood contamination. Three additional rectal secretion samples did not have sufficient IgG for quantitation at 1:5 dilution, so their denominator was replaced by ½ the LLOQ of their IgG ELISA runs. c IgG-normalized concentrations of VRC01 (pink) and VRC01LS (violet) in females who received a single 30 mg/kg IV infusion of VRC01 or VRC01LS. Serum and cervicovaginal secretions are depicted in individual graphs. Cervical and vaginal biopsies were not collected pre-infusion (timepoint 0); the baseline comparators (green interval line) are the 25–75 percentile ranges from pre-infusion samples from the other HVTN 116 study arms. Seven cervicovaginal secretions (n = 6 females; 8.9% of collections) did not contain sufficient fluid to run any assay, and 7 (n = 5 females; 8.9% of collections) had evidence of hemoglobin contamination; these samples were excluded. All mAb measurements in (b and c) are divided by the local concentration of IgG in the sample, and body weight-normalized. The p-values in (a–c) are two-sided Wilcoxon ranked sum tests adjusted for multiple comparisons using the Holm-Bonferroni method. Abbreviations mAb: monoclonal antibody; IgG immunoglobulin G; IQR: inter-quartile range; Cmax: Maximal concentration at 1-2 weeks post-infusion.