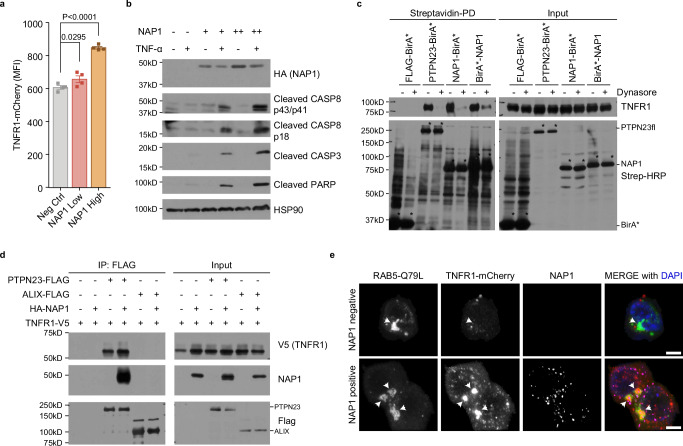
Fig. 7. NAP1 acts as an adapter protein in the regulation of TNFR1 signaling.
a Flow cytometric analysis of TNFR1-mCherry. The HEK 293 T cells stably expressing TNFR1-mCherry were transiently transfected with two doses of NAP1 constructs. Data are presented as mean ± SEM, n = 4 individual experiments. Statistics were determined by two-tailed, paired Welch’s t-test. b Immunoblotting analysis of cleaved CASP3, CASP8, and PARP in HEK 293 T cells. HEK 293 T cells were transiently transfected with increasing amounts of NAP1 plasmids. TNF-α (100 ng/ml) was added to fresh culture media 6 h post-transfection. Cells were harvested 24 h after TNF-α stimulation (n = 3 independent experiments). c Proximity labeling of TNFR1 by PTPN23-BirA* or NAP1-BirA* in the presence or absence of dynasore. HEK 293 T cells were transiently transfected with TNFR1 and Flag-BirA*, PTPN23-BirA*, NAP1-BirA*, or BirA*-NAP1. Biotin (50 μM) and dynasore (50 μM) were added into fresh media 6 h post-transfection, and incubated for 24 h. Asterisks mark the self-labeling of BirA* tagged recombinant proteins. d Co-immunoprecipitation of TNFR1 and NAP1 by FLAG-tagged PTPN23 or ALIX in HEK 293 T cells. Each immunoblot was reproduced three times with similar results for (c, d). e Representative confocal images of TNFR1-mCherry and eGFP-RAB5-Q79L in the presence or absence of NAP1. The HEK 293 T stably expressing TNFR1-mCherry cell line was transiently transfected with RAB5-Q79L and NAP1 plasmids. Cells were subjected to immunofluorescence staining 24 h after transfection. Scale bar: 5 μm.