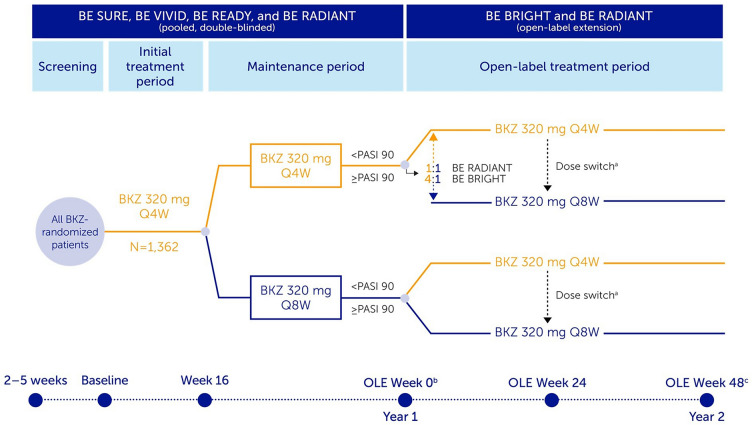
Fig. 1.
Study design. At baseline, 1362 patients with plaque psoriasis were randomized to bimekizumab (BKZ) across the included trials; 1107 of these patients received continuous BKZ and entered the open-label extensions (OLEs). In this analysis, BKZ every 4 weeks (Q4W) and every 8 weeks (Q8W) treatment arms shown are pooled for the BKZ total group. At OLE Week 0, patients receiving BKZ Q4W who achieved ≥ 90% improvement from baseline in Psoriasis Area and Severity Index (PASI 90) were re-randomized 4:1 in BE BRIGHT and 1:1 in BE RADIANT to open-label BKZ Q4W or Q8W; patients receiving BKZ Q8W who achieved PASI 90 at OLE Week 0 remained on Q8W dosing. Patients who did not achieve PASI 90 received open-label BKZ Q4W dosing. In BE READY, patients who did not achieve PASI 90 at Week 16, and patients who do not achieve ≥ 75% improvement from baseline in PASI (PASI 75) at any visit during the maintenance period, could enter a 12-week open-label BKZ 320 mg Q4W escape arm; if they achieved ≥ 50% improvement from baseline in PASI (50) at the end of the 12-week escape arm, they could then enter the BE BRIGHT OLE. Study designs for included trials have been published previously [16–19]. aDose switch: BE BRIGHT OLE Week 24, for patients achieving PASI 90 at investigator discretion; BE RADIANT OLE Week 16 or next scheduled clinic visit, dose switch added via protocol amendment; bAs a result of a lack of common visits across the studies, OLE Week 0 (the end of Year 1) corresponds to Week 48 for BE SURE, BE READY, and BE RADIANT, and Week 52 for BE VIVID;cOLE Week 48 (the end of Year 2) corresponds to BE RADIANT Week 96, BE VIVID/BE BRIGHT Week 100, and BE READY/BE BRIGHT and BE SURE/BE BRIGHT Week 104. BKZ bimekizumab, OLE open-label extension, PASI 90 ≥ 90% improvement from baseline in Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks