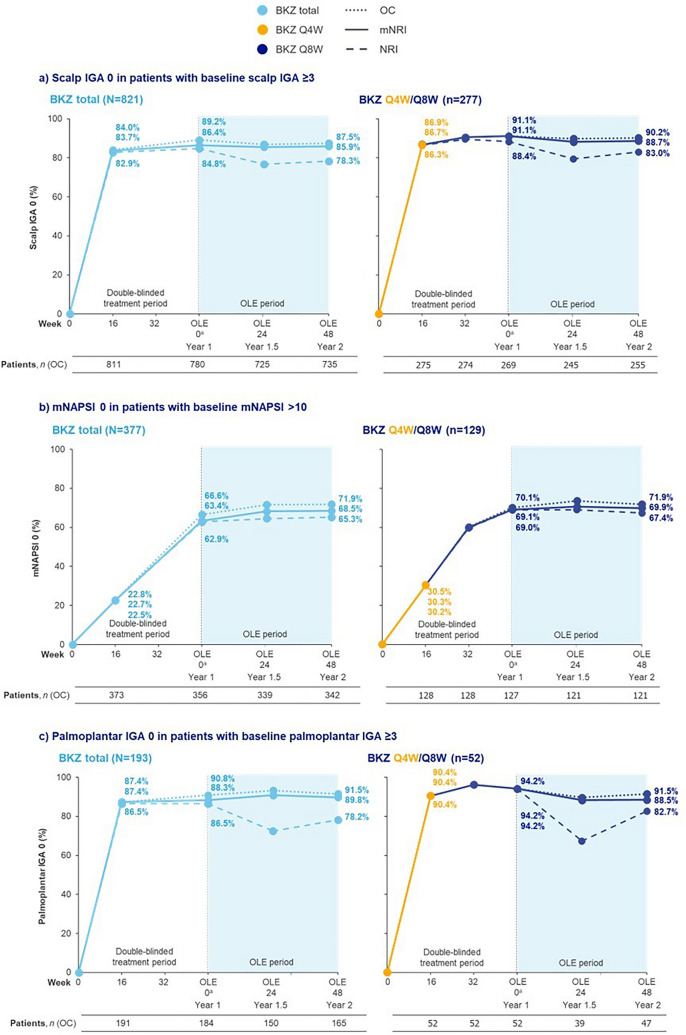
Fig. 2.
Complete clearance of high-impact areas over 2 years. Data are presented for patients with plaque psoriasis initially randomized to bimekizumab (BKZ) who later entered the open-label extension (OLE). For modified non-responder imputation (mNRI), patients with missing data following treatment discontinuation due to lack of efficacy or treatment-related adverse events were considered non-responders; multiple imputation methodology was used for all other missing data. BKZ total consists of patients randomized to receive BKZ 320 mg every 4 weeks (Q4W) to Week 16, received either BKZ Q4W or every 8 weeks (Q8W) to the end of the first year (Week 48/52/56), and entered the OLE. BKZ Q4W/Q8W consists of patients randomized to BKZ 320 mg Q4W to Week 16, who received BKZ Q8W throughout the maintenance period and on OLE entry; there are no BE VIVID patients in this treatment arm. Only visits common to all the studies included in a treatment arm are presented. aAs a result of a lack of common visits across the studies, OLE Week 0 corresponds to Week 48 for BE SURE, BE READY, and BE RADIANT, and Week 52 for BE VIVID. BKZ bimekizumab, IGA Investigator’s Global Assessment, mNAPSI modified Nail Psoriasis Severity Index, mNRI modified non-responder imputation, NRI non-responder imputation, OC observed case, OLE open-label extension, Q4W every 4 weeks, Q8W every 8 weeks