Abstract
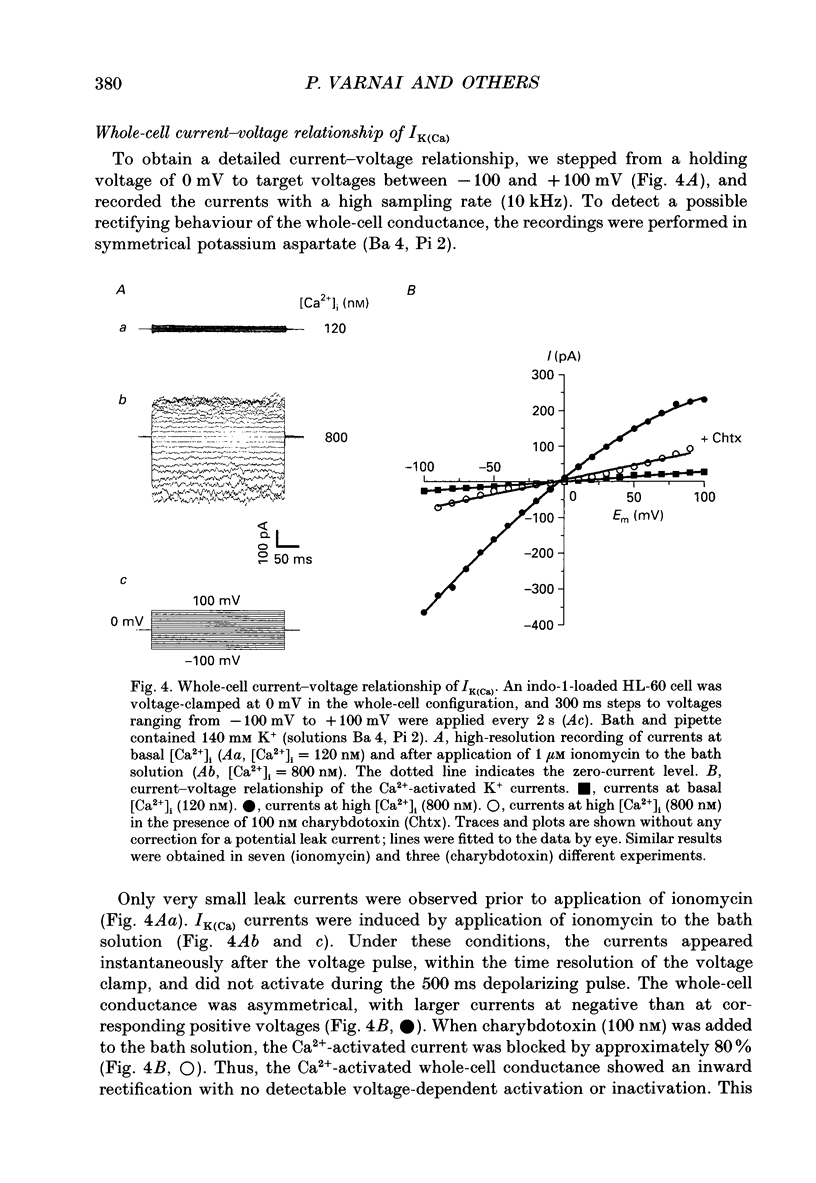
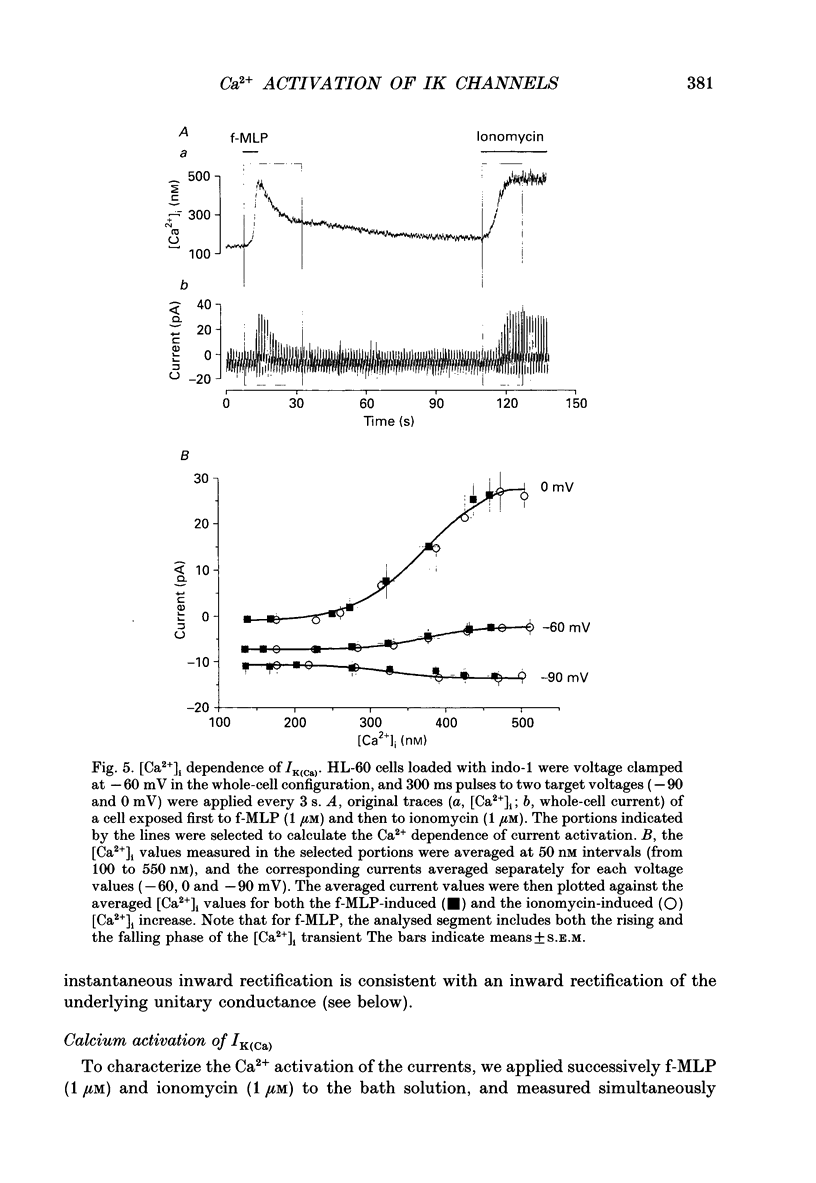
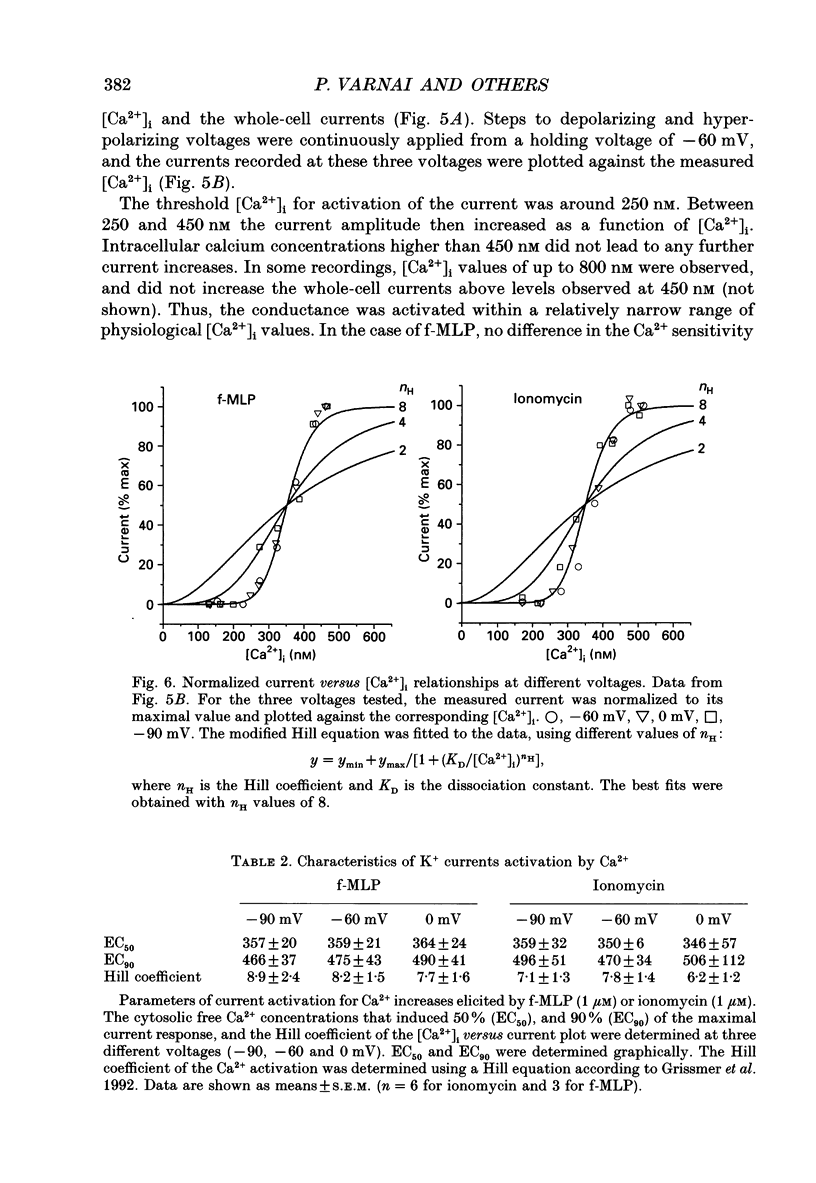
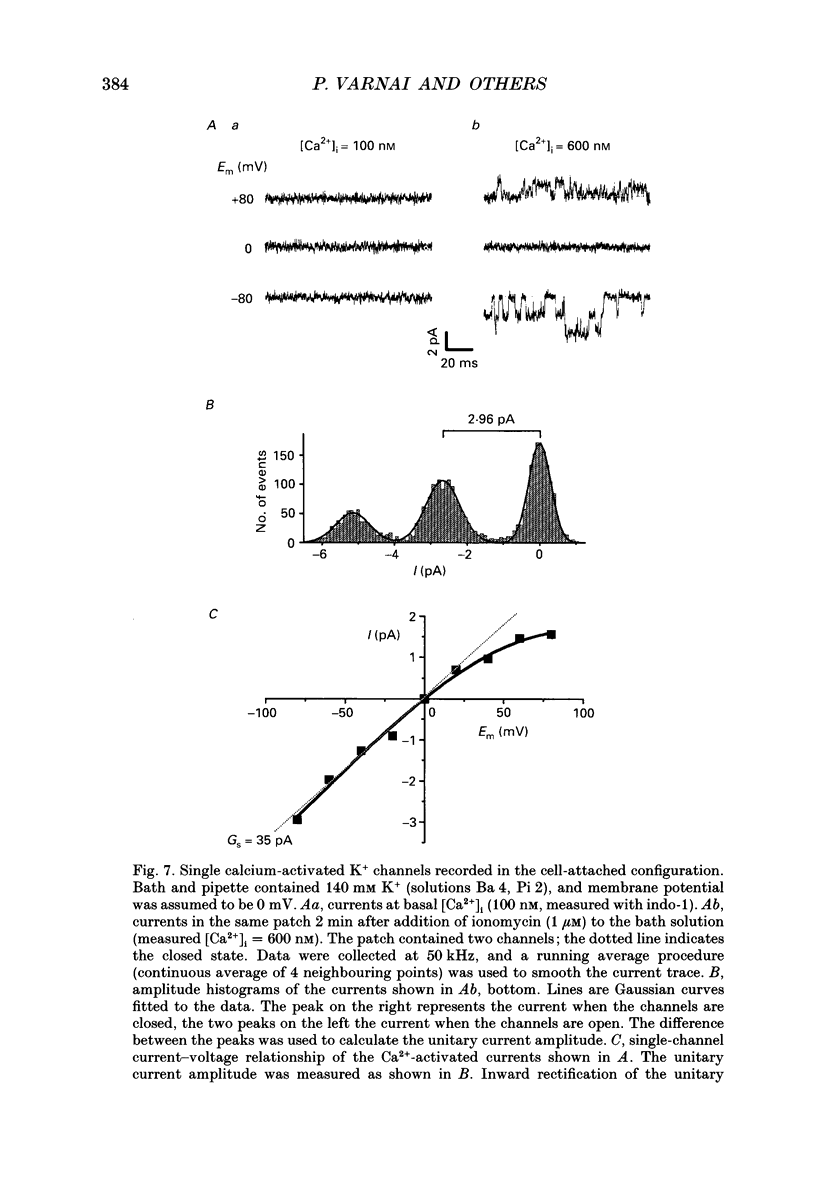
1. To study Ca(2+)-activated K+ currents in dimethyl sulphoxide (DMSO)-differentiated HL-60 cells (HL-60 granulocytes), we have combined the patch clamp technique with microfluorimetric measurements of the cytosolic free Ca2+ concentration ([Ca2+]i). 2. Elevations of [Ca2+]i induced by the receptor agonist N-formyl-L-methionyl-L-phenylalanine (f-MLP), by cellular spreading or by the Ca2+ ionophore ionomycin, activated whole-cell currents. The kinetics of the current elevations closely paralleled the kinetics of the elevations in [Ca2+]i. Cellular spreading induced oscillations in [Ca2+]i and parallel oscillatory changes in the amplitude of the recorded currents. 3. The reversal potential of the Ca(2+)-activated current was a function of the extracellular K+ concentration (56.1 mV per log [K+]), demonstrating that the underlying conductance was selective for K+. 4. The current was blocked by charybdotoxin, but insensitive to apamin. 5. The whole-cell current was inwardly rectifying. No time-dependent activation or inactivation of the current could be observed within the range of voltages tested (-100 to +100 mV). 6. The dependence of the current amplitude on the measured [Ca2+]i revealed a half-maximal activation at approximately 350 nM [Ca2+]i, and a highly co-operative activation by [Ca2+]i with an apparent Hill coefficient of approximately 8. Neither the half-maximal activation by [Ca2+]i nor the apparent Hill coefficient depended on the voltage, and they were identical for Ca2+ elevations caused by the ionophore and the receptor agonist. 7. Analysis of Ca(2+)-activated single-channel events in cell-attached recordings revealed an inwardly rectifying K+ channel with a slope conductance of 35 pS. Fluctuation analysis of the Ca(2+)-activated whole-cell current suggested an underlying single-channel conductance of a similar size (28 pS). 8. In summary, we describe a charybdotoxin-sensitive, intermediate-conductance Ca(2+)-activated K+ channel in HL-60 granulocytes. The characteristics of the Ca2+ activation of this current (i.e. sensitivity to submicromolar [Ca2+]i, high co-operativity and voltage independence) are similar to the Ca2+ activation of the apamin-sensitive small-conductance K+ channel. Our results also suggest that [Ca2+]i elevations are the predominant, if not the only, activators of this channel during physiological stimulation of HL-60 granulocytes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Grinstein S., Jaconi M., Schlegel W., Lew D. P., Krause K. H. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J Physiol. 1993 Jul;466:329–344. [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Schlegel W., Varnai P., Mayr G., Lew D. P., Krause K. H. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. J Clin Invest. 1992 Sep;90(3):830–839. doi: 10.1172/JCI115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew P. D., Andersson T., Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987 Apr 5;262(10):4574–4579. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gallin E. K. Calcium- and voltage-activated potassium channels in human macrophages. Biophys J. 1984 Dec;46(6):821–825. doi: 10.1016/S0006-3495(84)84080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K. Evidence for a Ca-activated inwardly rectifying K channel in human macrophages. Am J Physiol. 1989 Jul;257(1 Pt 1):C77–C85. doi: 10.1152/ajpcell.1989.257.1.C77. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Lewis R. S., Cahalan M. D. Ca(2+)-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992 Jan;99(1):63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R., Schwarz W., Passow H. Ca2+-activated K+ channels in human red cells. Comparison of single-channel currents with ion fluxes. Biophys J. 1984 Apr;45(4):693–698. doi: 10.1016/S0006-3495(84)84211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R., Schwarz W. Properties of the CA2+-activated K+ conductance of human red cells as revealed by the patch-clamp technique. Cell Calcium. 1983 Dec;4(5-6):499–510. doi: 10.1016/0143-4160(83)90025-8. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara N., Ichinose M., Sawada M., Imai K., Maeno T. Activation of single Ca2(+)-dependent K+ channel by external ATP in mouse macrophages. FEBS Lett. 1990 Jul 16;267(2):281–284. doi: 10.1016/0014-5793(90)80945-f. [DOI] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987 Sep 1;246(2):325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M. E., Theler J. M., Schlegel W., Appel R. D., Wright S. D., Lew P. D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991 Mar;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta Y., Okayama H., Aikawa T., Kanno T., Ohyama T., Sasaki H., Kato T., Takishima T. K channels of human alveolar macrophages. J Allergy Clin Immunol. 1988 Feb;81(2):460–468. doi: 10.1016/0091-6749(88)90918-9. [DOI] [PubMed] [Google Scholar]
- Kanno T., Takishima T. Chloride and potassium channels in U937 human monocytes. J Membr Biol. 1990 Jun;116(2):149–161. doi: 10.1007/BF01868673. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Welsh M. J. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990 Feb;85(2):491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Lew V. L., Muallem S., Seymour C. A. The Ca-activated K channel of human red cells: all or none behaviour of the Ca2+-gating mechanism. Cell Calcium. 1983 Dec;4(5-6):511–517. doi: 10.1016/0143-4160(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Mason M. J. Ca(2+)-activated K+ channels in rat thymic lymphocytes: activation by concanavalin A. J Physiol. 1991 Aug;439:513–528. doi: 10.1113/jphysiol.1991.sp018679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Schlichter L. C. Ca2(+)-activated K+ channels in human B lymphocytes and rat thymocytes. J Physiol. 1989 Aug;415:69–83. doi: 10.1113/jphysiol.1989.sp017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Nauseef W. M., Clark R. A. Depolarization blunts the oxidative burst of human neutrophils. Parallel effects of monoclonal antibodies, depolarizing buffers, and glycolytic inhibitors. J Immunol. 1988 Jun 1;140(11):3928–3935. [PubMed] [Google Scholar]
- McCann F. V., Keller T. M., Guyre P. M. Ion channels in human macrophages compared with the U-937 cell line. J Membr Biol. 1987;96(1):57–64. doi: 10.1007/BF01869334. [DOI] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Grinstein S. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10816–10820. doi: 10.1073/pnas.88.23.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D., Di Virgilio F., Pozzan T., Monod A., Lew D. P. Correlation between plasma membrane potential and second messenger generation in the promyelocytic cell line HL-60. J Biol Chem. 1990 Aug 25;265(24):14256–14263. [PubMed] [Google Scholar]
- Toro L., Stefani E. Calcium-activated K+ channels: metabolic regulation. J Bioenerg Biomembr. 1991 Aug;23(4):561–576. doi: 10.1007/BF00785811. [DOI] [PubMed] [Google Scholar]
- Wieland S. J., Gong Q. H., Chou R. H., Brent L. H. A lineage-specific Ca(2+)-activated K+ conductance in HL-60 cells. J Biol Chem. 1992 Aug 5;267(22):15426–15431. [PubMed] [Google Scholar]
- Wolff D., Cecchi X., Spalvins A., Canessa M. Charybdotoxin blocks with high affinity the Ca-activated K+ channel of Hb A and Hb S red cells: individual differences in the number of channels. J Membr Biol. 1988 Dec;106(3):243–252. doi: 10.1007/BF01872162. [DOI] [PubMed] [Google Scholar]


