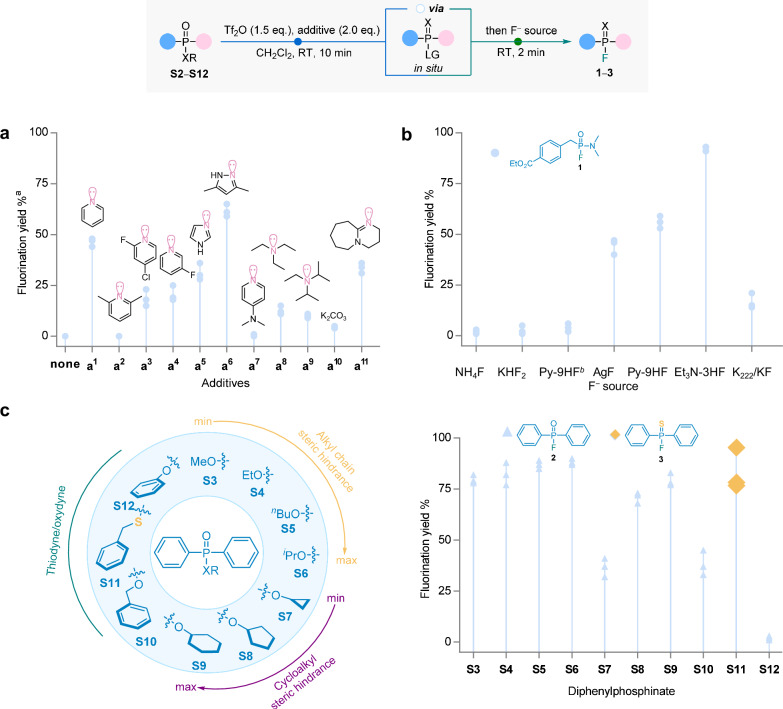
Fig. 2. Optimization of conditions for late-stage fluorination of alkyl phosphonates via electrophilic activation.
All reactions were conducted using 0.2 mmol model compounds, with a gradual addition of Tf2O (1.5 eq., 0.3 mmol) and the additive (2.0 eq., 0.4 mmol), followed by the subsequent addition of the F− source (1.5 eq., 0.3 mmol), in CH2Cl2 (1.0 mL, 0.2 M) under room temperature (RT). a Optimization of bases, ethyl 4-(((dimethylamino)(ethoxy)phosphoryl)methyl)benzoate (S2) as a model compound. aYields determined by 31P NMR. 31P NMR was conducted by adding CD₂Cl₂ after the reaction, with the preliminary conversion proportion calculated from the ratio of product to non-product peak areas. b Screening of fluorine sources with S2 as the model compound and Py as an additive. bNo additive is added. c, Study of direct fluorination of different diphenylphosphonates (S3−S12).