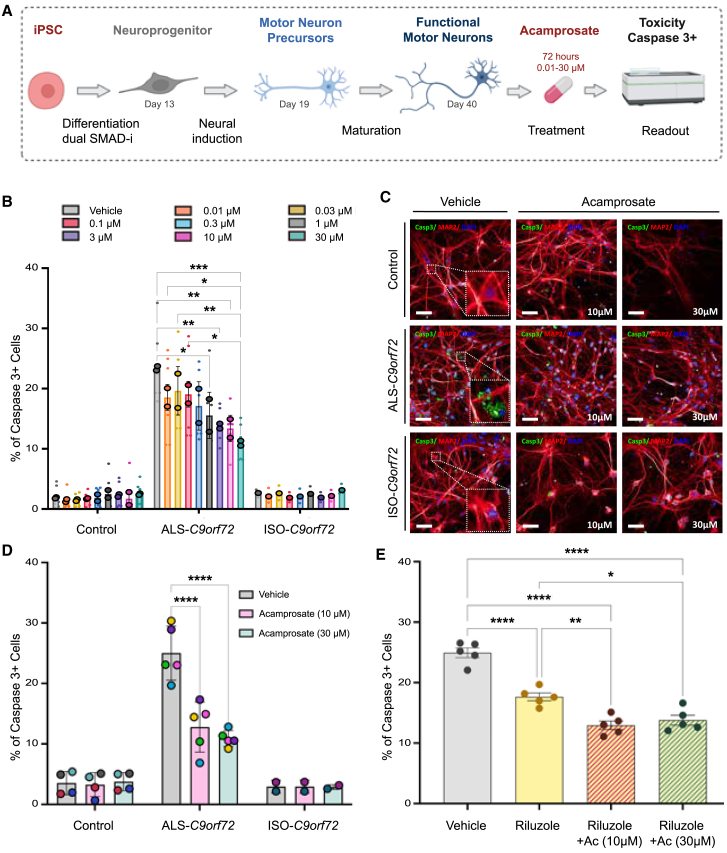
Figure 6.
Acamprosate is neuroprotective in iPSC-derived motor neurons from C9orf72 patients
(A) Schematic representation of the experiments to validate the effect of acamprosate.
(B) The bar graph depicts the percentage of cells showing cleaved caspase-3 (caspase-3+ cells) after acamprosate treatment. Minor dots represent biological replicates, averaging from three technical replicates each. In contrast, the bordered dots represent the mean effect in iPSC-derived motor neurons from two healthy donors, two C9orf72 ALS patients, and one isogenic line. Data are mean ± SD. Comparisons within the control and the ALS-C9orf72 groups were performed using a two-way ANOVA, with a Tukey’s post hoc test (n = 2). Only p < 0.05 and comparisons with the vehicle group are displayed in the graph.
(C) Representative images of the motor neurons showing cleaved caspase-3 staining (green), MAP2 staining (red), and DAPI (blue). Scale bar, 50 μm. All molecular phenotypes were confirmed in a minimum of 3 technical replicates, and at least 25 fields were randomly selected and scanned per well of a 96-well plate in triplicate.
(D) Acamprosate effective doses (10 and 30 μM) were confirmed in additional iPSC-derived motor neurons, totaling cells from four healthy donors, five C9orf72 ALS patients, and two isogenic lines. Each point represents the mean effect per cell line. Data are mean ± SD. Comparisons within the control, the ALS-C9orf72, and the ISO-C9orf72 groups were performed using a two-way ANOVA, with Tukey’s post hoc test (control, n = 4; ALS-C9orf72, n = 5; ISO-C9orf72, n = 2). p < 0.05 are annotated.
(E) The graph shows the percentage of cleaved caspase-3+ motor neurons derived from C9orf72 patients treated with riluzole (10 μM) and riluzole plus acamprosate (10 and 30 μM). Dots represent the mean effect of each line. Data are mean ± SD. Comparisons were performed using a one-way ANOVA with Tukey’s post hoc test (n = 5). p < 0.05 are annotated as follows: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.001, ∗∗∗∗∗p < 0.0001.
See also Figures S6–S9 and Table S10.