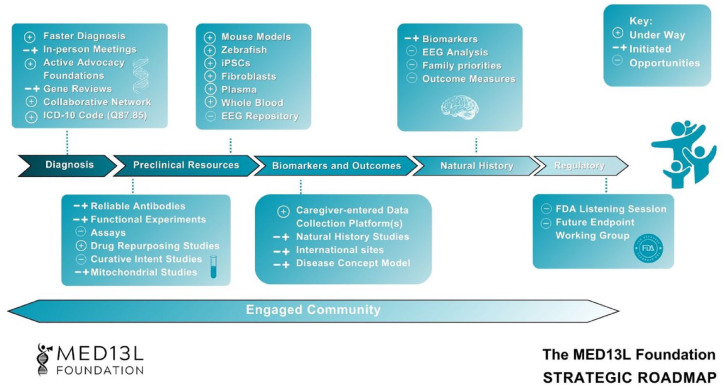
Figure 1.
The MED13L foundation strategic roadmap.5–7 The roadmap serves as a summary of progress regarding the necessary preclinical trial tools and resources developed as part of the larger SRP. Each forward-moving arrow represents a broad category of needs, from diagnosis to regulatory tasks, essential for optimal clinical trial readiness. Each box above and below the arrows represents a set of tools and resources that align with these categories. The resources may flow across arrows and do not fully represent all that must be achieved to reach clinical trials or therapeutic development. Additionally, tools and resources may apply to many rare disorders or be specific to a particular disorder. An engaged and active community is crucial throughout the entire process. Early and intentional investment in individuals affected by MED13L syndrome is key to developing effective therapeutics.
EEG, electroencephalogram; FDA, Food and Drug Administration; ICD-10, International Classification of Diseases, 10th revision; iPSCs, induced pluripotent stem cells; SRP, strategic research plan.