Abstract
Objectives
This study aimed to examine the prevalence of type 2 diabetes (T2D) and the independent and joint associations of sleep duration and different volumes of physical activity (PA) with T2D in the China Health and Retirement Longitudinal Study (CHARLS).
Methods
The prevalence of T2D among the Chinese population aged 45 years and older was estimated for the years 2011, 2013, 2015, 2018, and 2020. Data from 2020 were used to examine the independent and joint associations of sleep duration and different volumes of PA with T2D. Sleep duration was classified into three categories: short (< 6 h/day), normal (6–8 h/day), and long (> 8 h/day). PA volumes were classified based on the IPAQ recommendations as follows: light-volume PA (LPA, < 600 MET-minutes/week), moderate-volume PA (MPA, 600–3000 MET-minutes/week), and vigorous-volume PA (VPA, > 3000 MET-minutes/week). The data were statistically analyzed using a t-test and analysis of variance (ANOVA). Multivariate logistic regression models were used to examine the independent and joint associations of PA and sleep duration with T2D.
Results
The prevalence of T2D in the LPA and short sleep groups increased from 13.35% (95% CI = 10.41–16.75) and 11.52% (95% CI = 10.01–13.15) in 2011 to 17.27% (95% CI = 15.09–19.62) and 16.28% (95% CI = 15.34–17.25) in 2020, respectively. Compared with LPA, VPA was associated with lower odds of T2D (Model 3, OR = 0.82, 95% CI = 0.69–0.97). Compared to individuals with normal sleep duration, those with short sleep duration had a higher likelihood of T2D (Model 3, OR = 1.10, 95% CI = 1.08–1.22), whereas long sleep duration did not show a significant association (Model 3, OR = 1.03, 95% CI = 0.86–1.23). The risk of developing T2D was approximately 35% lower for individuals with LPA and normal sleep duration compared to those with LPA and short sleep duration (Model 3, OR = 0.65, 95% CI = 0.46–0.91). In the VPA group, the mitigation effect of exercise on T2D was observed regardless of sleep duration (Model 3, short: OR = 0.73, 95% CI = 0.56–0.95; normal: OR = 0.65, 95% CI = 0.51–0.85; long: OR = 0.63, 95% CI = 0.45–0.89).
Conclusions
The prevalence of T2D among middle-aged and older adults in China increased substantially from 2011 to 2020. Short sleep duration is associated with higher odds of developing T2D. However, engaging in VPA mitigates this risk, even in those with insufficient sleep.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20743-y.
Keywords: Type 2 diabetes, Light-volume physical activity, Moderate-volume physical activity, Vigorous-volume physical activity, China Health and Retirement Longitudinal Study
Introduction
Type 2 diabetes (T2D) accounts for approximately 90% of all diabetes cases [1] and is a leading cause of disability and mortality worldwide [2]. T2D interacts with and exacerbates many other diseases [3]. The burden of T2D can largely be attributed to modifiable social risk factors [3], including physical inactivity [4], disrupted sleep patterns [5], prolonged sedentary behavior [6], dietary risks [7], and environmental and occupational risks [1]. Poor sleep quality, characterized as sleep duration outside 6–8 h, difficulty sleeping or waking, or frequent disturbances, adversely impacts the development of diabetes through various mechanisms [8, 9]. Self-reported short and long sleep durations (outside the 6–8 h range) are associated with an increased risk of T2D [10]. Therefore, screening for sleep quality, particularly short and long sleep duration, is recommended as a preventive measure for T2D.
Increased physical activity (PA) has been associated with lower hemoglobin A1c (HbA1c) levels [11] and decreased risk of T2D [12]. As such, PA has been identified in the American Diabetes Association guidelines as a target for preventing and managing T2D [13]. The benefits of vigorous-volume PA (VPA) are well established [14], whereas evidence regarding moderate-volume PA (MPA) and light-volume PA (LPA) remains controversial [15]. PA and sleep are interrelated, with higher PA volume associated with better sleep quality. They may also influence T2D through distinct mechanisms [16, 17]. The relationship between these two factors is highlighted by the notion that improving one behavior can enhance the other [18]. Evidence also suggests causal and therapeutic associations between PA and sleep quality [19]. However, few studies have examined the combined association of PA and sleep with T2D. Jin et al. [20] investigated the association between accelerometer-measured sleep duration and different PA intensities with the risk of incident T2D using data from the UK Biobank. The findings suggest that increased levels of PA, regardless of intensity, may help prevent the development of T2D in individuals with short sleep duration. However, there are significant differences in PA levels [21] and sleep duration [22] across different countries and regions, which may influence the incidence and prevention strategies for T2D. Therefore, it is essential to further investigate the joint effects of PA and sleep duration on T2D within specific national or regional contexts to develop more effective public health policies and interventions.
This study aimed to investigate the independent associations of PA and sleep duration with T2D, as well as how these factors interact to play a role in T2D, using data from the China Health and Retirement Longitudinal Study (CHARLS). We compared the prevalence and odds ratio of T2D across different groups based on PA and sleep duration in the Chinese population.
Methods
Study design and population
CHARLS is an ongoing longitudinal survey that investigates the social, economic, and health conditions of the Chinese population aged 45 and above [23]. The baseline survey, employing a multi-stage probability sampling strategy, commenced in 2011 (Wave 1), with subsequent follow-up surveys conducted in 2013 (Wave 2), 2015 (Wave 3), 2018 (Wave 4), and 2020 (Wave 5). The CHARLS questionnaire includes various content areas such as personal demographics, family structure and economic support, health status, physical measurements, healthcare utilization and insurance, employment, retirement and pensions, income, consumption, assets, and basic community characteristics. For detailed information on the objectives, design, sample, and questionnaires of CHARLS, see Supplementary file and Zhao et al. [23]. CHARLS has received ethical approval from the Institutional Review Board of Peking University.
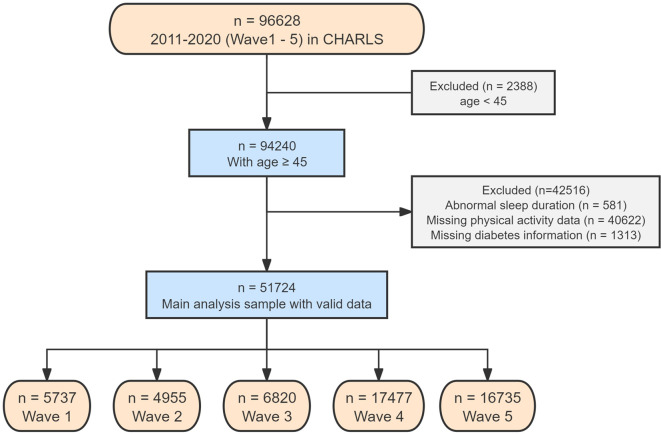
We used data from all five years (Wave 1 to Wave 5) to assess changes in T2D prevalence. Several exclusion criteria were applied to refine the study population. First, we excluded 2,388 individuals aged less than 45 years and 581 individuals with abnormal sleep durations. Additionally, we excluded 40,622 participants who lacked PA measurements and 1,313 participants with missing T2D information. After applying these criteria, our final analysis encompassed 51,724 participants: 5,737 in 2011, 4,955 in 2013, 6,820 in 2015, 17,477 in 2018, and 16,735 in 2020. Furthermore, to explore independent and joint relationships, we selected an additional 16,735 participants from the Wave 5 (2020) survey. The detailed methodology of the participant selection process is illustrated in Fig. 1.
Fig. 1.
Flowchart of participant enrollment
Exposure
The exposures in the current study included questionnaire-measured sleep duration and PA. The raw data were processed by the CHARLS working group. The included participants were asked about the intensity, duration, and frequency (days per week) of their PA, as well as their typical daily sleep duration.
The PA questionnaire was derived from pages 70–71 of the CHARLS questionnaire. The volume of PA was calculated using the corresponding metabolic equivalent (MET) [24] values and IPAQ scoring standards [25]. CHARLS did not collect the precise duration of PA from respondents; instead, it inquired about PA in interval categories. Based on the questionnaire responses, we classified daily PA duration into 5 groups: 0 min, 10–29 min, 30–119 min, 120–239 min, and ≥ 240 min, using the midpoint values for analysis. PA types were further classified into VPA (MET = 8.0, e.g., climbing, running, farming), MPA (MET = 4.0, e.g., brisk walking, Tai Chi), and LPA (MET = 3.3, e.g., casual walking) based on their corresponding MET values. MET-minutes/week = MET value * days * duration [26]. According to IPAQ standards, weekly PA levels were categorized into LPA (< 600 MET-minutes/week), MPA (600–3000 MET-minutes/week), and VPA (> 3000 MET-minutes/week). To identify outliers in PA volume, we calculated the first quartile (Q1) and third quartile (Q3) of the data. We then used the Interquartile Range (IQR) method to detect outliers: values below Q1 – 1.5 * IQR or above Q3 + 1.5 * IQR were considered potential outliers.
In the CHARLS, self-reported nighttime sleep duration was obtained through the question: “During the past month, how many hours of actual sleep did you get at night?” Daytime napping was assessed through the question: “During the past month, for how long did you take a nap after lunch?” In this study, self-reported sleep duration was calculated as the sum of nighttime sleep and daytime napping hours per day. Following prior studies [27] and the consensus recommendations of the American Academy of Sleep Medicine [28], participants were divided into three groups based on sleep duration: short (< 6 h/day), normal (6–8 h/day), and long (> 8 h/day). Participants were also excluded if their mean sleep duration was less than 3 or longer than 11 h [20].
Covariates
The following variables were considered likely confounding factors: gender (male/female), marital status (marry or others), education (less than primary/primary school/middle school/high school and above), smoking and drinking status (yes or no), retirement status (with/without), residential area (urban or rural), household size (number of members) and other diabetes-related comorbidities (hypertension, cancer, stomach diseases, liver diseases, kidney diseases).
Statistics analysis
Baseline characteristics are presented as the mean (standard deviation, SD) for continuous variables or number (percentage) for categorical variables, as appropriate. At baseline, we used Pearson’s chi-square test (for homogeneous variances) or Fisher’s exact test (for heterogeneous variances) to analyze the differences in categorical variables across PA or sleep duration groups. For continuous variables, we employed one-way analysis of variance (ANOVA, for homogeneous variances) or the Kruskal-Wallis test (for heterogeneous variances) to examine the differences in the prevalence of T2D among the different PA and sleep duration categories.
We employed three multivariate logistic regression models to estimate the independent associations of PA (LPA, MPA, and VPA) and sleep duration (short, normal, and long) with T2D. Model 1 was adjusted for age and sex. Model 2 was further adjusted for marital status, education level, smoking and drinking status, residential area, retirement status, and household size based on Model 1. Model 3 was adjusted for Model 2 plus hypertension, cancer, stomach diseases, liver diseases, and kidney diseases.
To assess the joint association between PA and sleep duration, we first examined the interactions between sleep duration and PA in relation to the odds of T2D on both additive and multiplicative scales. Second, we subdivided the entire sample into nine groups based on PA volume and sleep duration categories. We then used multivariable logistic regression models to estimate the joint association of PA and sleep duration with T2D, with the group having LPA and short sleep duration serving as a reference. All statistical analyses were performed using R studio software version 4.2, and statistical significance was defined as p < 0.05.
Results
Characteristics
Table 1 presents the prevalence of T2D across different levels of PA and sleep duration between 2011 and 2020. The overall prevalence of T2D across the groups was as follows: LPA, 15.01%; MPA, 16.17%; VPA, 11.66%; short sleep, 14.33%; normal sleep, 12.49%; and long sleep, 13.03%. Among these groups, the VPA (11.66%) and normal sleep (12.49%) groups had the lowest prevalence of T2D.
Table 1.
The prevalence of T2D across different levels of PA and sleep duration between 2011 and 2020
| LPA | MPA | VPA | Short sleep | Normal sleep | Long sleep | ||
|---|---|---|---|---|---|---|---|
| 2011 | Events/n | 63 | 169 | 432 | 190 | 422 | 52 |
|
Prevalence (95% CI) |
13.35% (10.41–16.75) |
12.88% (11.11–14.81) |
10.93% (9.97–11.94) |
11.52% (10.01–13.15) |
11.58% (10.56–12.66) |
11.76% (8.91–15.14) |
|
| 2013 | Events/n | 34 | 125 | 173 | 103 | 201 | 28 |
|
Prevalence (95% CI) |
8.52% (5.97–11.70) |
9.54% (8.01–11.26) |
5.33% (4.58–6.16) |
6.56% (5.38–7.90) |
6.63% (5.77–7.58) |
7.93% (5.33–11.26) |
|
| 2015 | Events/n | 93 | 330 | 708 | 356 | 674 | 101 |
|
Prevalence (95% CI) |
19.42% (15.97–23.25) |
19.35% (17.50-21.31) |
15.27% (14.24–16.33) |
16.85% (15.28–18.51) |
16.61% (15.47–17.78) |
15.59% (12.87–18.61) |
|
| 2018 | Events/n | 167 | 810 | 1243 | 837 | 1183 | 200 |
|
Prevalence (95% CI) |
13.96% (12.05–16.06) |
16.40% (15.37–17.46) |
10.96% (10.39–11.55) |
14.37% (13.48–15.29) |
11.74% (11.11–12.38) |
12.71% (11.10-14.45) |
|
| 2020 | Events/n | 192 | 876 | 1382 | 950 | 1337 | 163 |
|
Prevalence (95% CI) |
17.27% (15.09–19.62) |
17.47% (16.42–18.55) |
13.03% (12.39–13.68) |
16.28% (15.34–17.25) |
13.73% (13.05–14.43) |
14.06% (12.11–16.20) |
|
| Total | Events/n | 549 | 2310 | 3938 | 2436 | 3817 | 544 |
|
Prevalence (95% CI) |
15.01% (13.88–16.18) |
16.17% (15.57–16.79) |
11.66% (11.32–12.01) |
14.33% (13.77–14.91) |
12.49% (12.14–12.84) |
13.03% (12.06–14.05) |
|
| Trends | P for trends | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
Abbreviations 95% CI = 95% confidence interval; LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity
Table 2 shows the baseline characteristics of the study participants stratified by PA volume in 2020. A total of 16,735 participants were included, with a mean age of 62.83 ± 9.52 years and 52.35% females. Of these, 1,112 were in the LPA group (mean age, 66.63 ± 10.98 years; 55.58% females), 5,015 were in the MPA group (mean age, 64.13 ± 9.90 years; 52.08% females), and 10,608 were in the VPA group (mean age, 61.81 ± 8.98; 52.14% females). The percentages of T2D in the LPA, MPA, and VPA groups was 6.64%, 29.96%, and 63.4%, respectively. More than half of the participants engaged in moderate to vigorous PA (MVPA) levels. Compared to VPA participants, those with LPA were more likely to report a history of dyslipidemia, hypertension, heart disease, cancer, lung disease, liver disease, stroke, mental disorder, stomach disease, and asthma (p < 0.05, Supplementary Table S1), whereas MPA tended to have a history of dyslipidemia, hypertension, heart disease, cancer, stroke, kidney disease, mental disorder, and asthma (p < 0.05, Supplementary Table S1).
Table 2.
Baseline characteristics of the study participants stratified by PA volume (n = 16735) in 2020, data are presented as mean ± SD or n (%)
| Characteristic | LPA (n = 1112) |
MPA (n = 5015) |
VPA (n = 10608) |
Total (n = 16735) |
χ² P |
|---|---|---|---|---|---|
| Age (years) | 66.63 ± 10.98 | 64.13 ± 9.90 | 61.81 ± 8.98 | 62.83 ± 9.52 | 617.73 (p < 0.001) |
|
Male Female |
494 (44.42%) 618 (55.58%) |
2403 (47.92%) 2612 (52.08%) |
5077 (47.86%) 5531(52.14%) |
7974 (47.65%) 8761 (52.35%) |
4.97 (p = 0.083) |
|
Sleep duration Short (hours/day) Normal (hours/day) Long (hours/day) |
407 (36.60%) 608 (54.68%) 97 (8.72%) |
1719 (34.28%) 2991 (59.64%) 305 (6.08%) |
3711 (34.98%) 6140 (57.88%) 757 (7.14%) |
5837 (34.88%) 9739 (58.20%) 1159 (6.93%) |
16.87 (p < 0.05) |
|
Education Less than Primary Primary School Middle School High School and Above |
498 (44.78%) 247 (22.21%) 222 (19.96%) 145 (13.04%) |
1722 (34.34%) 1112 (22.17%) 1236 (24.65%) 945 (18.84%) |
4588(43.25%) 2372 (22.36%) 2411 (22.73%) 1237 (11.66%) |
6808 (40.68%) 3731 (22.29%) 3869 (23.12%) 2327 (13.90%) |
209.00 (p < 0.001) |
| Marry | 868 (78.06%) | 4157 (82.89%) | 9289 (87.57%) | 14,314 (85.53%) | 113.93 (p < 0.001) |
| Smoke | 263 (23.65%) | 1208 (24.09%) | 2841 (26.78%) | 4312 (25.77%) | 15.73 (p < 0.001) |
| Drink | 363 (32.64%) | 1767 (35.23%) | 4133 (38.96%) | 6263 (37.43%) | 31.87 (p < 0.001) |
| Type 2 Diabetes | 192 (17.27%) | 876 (17.47%) | 1382 (13.03%) | 2450 (14.64%) | 60.28 (p < 0.001) |
| Dyslipidemia | 381 (34.26%) | 1529 (30.49%) | 2550 (24.04%) | 4460 (26.65%) | 107.78 (p < 0.001) |
| Hypertension | 502 (45.14%) | 2169 (43.25%) | 3834 (36.14%) | 6505 (38.87%) | 92.12 (p < 0.001) |
| Heart Disease | 315 (28.33%) | 1153 (22.99%) | 1910 (18.01%) | 3378 (20.19%) | 101.55 (p < 0.001) |
| Cancer | 41 (3.69%) | 160 (3.19%) | 216 (2.04%) | 417 (2.49%) | 25.68 (p < 0.001) |
| Lung Disease | 200 (17.99%) | 711 (14.18%) | 1420 (13.39%) | 2331 (13.93%) | 18.13 (p < 0.001) |
| Liver Disease | 96 (8.63%) | 389 (7.76%) | 738 (6.96%) | 1223 (7.31%) | 6.30 (p < 0.05) |
| Stroke | 104 (9.35%) | 416 (8.30%) | 548 (5.17%) | 1068 (6.38%) | 73.40 (p < 0.001) |
| Kidney Disease | 131 (11.78%) | 568 (11.33%) | 1052 (9.92%) | 1751 (10.46%) | 9.42 (p < 0.05) |
| Mental Disorder | 55 (4.95%) | 150 (2.99%) | 233 (2.20%) | 438 (2.62%) | 33.78 (p < 0.001) |
| Stomach Disease | 403 (36.24%) | 1604 (31.98%) | 3346 (31.54%) | 5353 (31.99%) | 10.21 (p < 0.05) |
| Asthma | 109 (9.80%) | 303 (6.04%) | 527 (4.97%) | 939 (5.61%) | 46.92 (p < 0.001) |
Note Percentages add up not to 100% due to rounding
Abbreviations LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity
Table 3 shows the baseline characteristics of the study participants stratified by sleep duration in 2020. The percentages of participants with short, normal, and long sleep duration were 34.8%, 58.2%, and 6.9%, respectively. Approximately 40% of the study population did not meet the recommended sleep duration guidelines, indicating that insufficient sleep was a prevalent issue. Compared to participants with normal sleep duration, those with short sleep duration were more likely to have a history of dyslipidemia, hypertension, heart disease, cancer, lung disease, liver disease, stroke, kidney disease, mental disorder, stomach disease, and asthma (p < 0.05, Supplementary Table S2), whereas long sleepers tended to have a history of dyslipidemia, hypertension, lung disease, stroke, and asthma (p < 0.05, Supplementary Table S2).
Table 3.
Baseline characteristics of the study participants stratified by sleep duration (n = 16735) in 2020, data are presented as mean ± SD or n (%)
| Characteristic | Short (n = 5837) |
Normal (n = 9739) |
Long (n = 1159) |
Total (n = 16735) |
χ² P |
|---|---|---|---|---|---|
| Age (years) | 64.11 ± 9.38 | 61.63 ± 9.30 | 66.45 ± 10.20 | 62.83 ± 9.52 | 629.70 (p < 0.001) |
|
Male Female |
2403 (41.17%) 3434 (58.83%) |
4973 (51.06%) 4766 (48.94%) |
598 (51.60%) 561 (48.40%) |
7974 (47.65%) 8761 (52.35%) |
151.01 (p < 0.001) |
|
LPA MPA VPA |
407(6.97%) 1719 (29.45%) 3711 (63.58%) |
608 (6.24%) 2991 (30.71%) 6140 (63.05%) |
97 (8.37%) 305 (26.32%) 757 (65.31%) |
1112 (6.64%) 5015 (29.97%) 10,608 (63.39%) |
16.87 (p < 0.05) |
|
Education Less than Primary Primary School Middle School High School and Above |
2743 (46.99%) 1274 (21.83%) 1179 (20.20%) 641 (10.98%) |
3423 (35.15%) 2207 (22.66%) 2509 (25.76%) 1600 (16.43%) |
642 (55.39%) 250 (21.57%) 181 (15.62%) 86 (7.42%) |
6808 (40.68%) 3731 (22.29%) 3869 (23.12%) 2327 (13.90%) |
388.29 (p < 0.001) |
| Marry | 4802 (82.27%) | 8575 (88.05%) | 937 (80.85%) | 14,314 (85.53%) | 120.64 (p < 0.001) |
| Smoke | 1317 (22.57%) | 2668 (27.40%) | 327 (28.21%) | 4312 (25.77%) | 48.37 (p < 0.001) |
| Drink | 2014 (34.51%) | 3874 (39.78%) | 375 (32.36%) | 6263 (37.43%) | 56.92 (p < 0.001) |
| Type 2 Diabetes | 950 (16.28%) | 1337 (13.73%) | 163 (14.06%) | 2450 (14.64%) | 19.27 (p < 0.001) |
| Dyslipidemia | 1761 (30.17%) | 2461 (25.27%) | 238 (20.53%) | 4460 (26.65%) | 68.65 (p < 0.001) |
| Hypertension | 2434 (41.70%) | 3575 (36.71%) | 496 (42.80%) | 6505 (38.87%) | 46.34 (p < 0.001) |
| Heart Disease | 1468 (25.15%) | 1691 (17.36%) | 219 (18.90%) | 3378 (20.19%) | 138.63 (p < 0.001) |
| Cancer | 179 (3.07%) | 214 (2.20%) | 24 (2.07%) | 417 (2.49%) | 12.26 (p < 0.001) |
| Lung Disease | 1037 (17.77%) | 1121 (11.51%) | 173 (14.93%) | 2331 (13.93%) | 120.16 (p < 0.001) |
| Liver Disease | 540 (8.63%) | 621 (7.76%) | 62 (6.96%) | 1223 (7.31%) | 51.58 (p < 0.001) |
| Stroke | 483 (8.27%) | 489 (5.02%) | 96 (8.28%) | 1068 (6.38%) | 72.20 (p < 0.001) |
| Kidney Disease | 786 (13.47%) | 878 (9.02%) | 87 (7.51%) | 1751 (10.46%) | 88.78 (p < 0.001) |
| Mental Disorder | 217 (3.72%) | 189 (1.94%) | 32 (2.76%) | 438 (2.62%) | 45.31 (p < 0.001) |
| Stomach Disease | 2287 (39.18%) | 2748 (28.22%) | 318 (27.44%) | 5353 (31.99%) | 213.53 (p < 0.001) |
| Asthma | 446 (7.64%) | 415 (4.26%) | 78 (6.73%) | 939 (5.61%) | 81.66 (p < 0.001) |
Note Percentages add up not to 100% due to rounding
Abbreviations LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity
Change in the prevalence of T2D across varying volumes of PA and sleep duration between 2011 and 2020
The prevalence of T2D among middle-aged and older adults with LPA in China increased by 3.92% between 2011 and 2020, rising from 13.35 to 17.27% (p < 0.05 for trend; Fig. 2), representing a 29.33% relative increase. The prevalence of T2D among middle-aged and older adults with MPA increased by 4.59%, from 12.88 to 17.47% (p < 0.001 for trend), equating to a 35.65% relative increase. In contrast, those with VPA showed a smaller increase from 10.93 to 13.03% (p < 0.001 for trend).
Fig. 2.
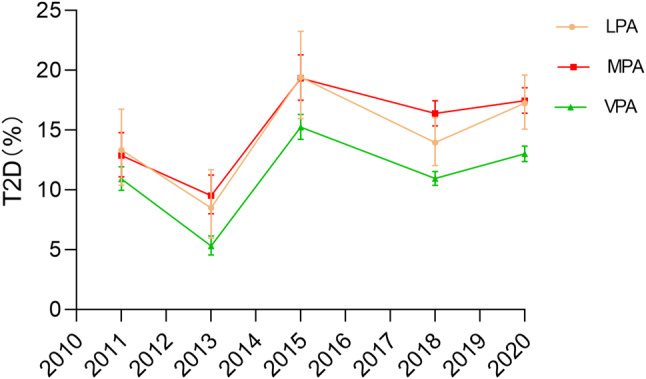
Variation in T2D prevalence over the years across different volumes of PA
Similarly, the prevalence of T2D increased by 4.76% among middle-aged and older adults with short sleep duration (11.52% in 2011 to 16.28% in 2020, p < 0.001 for trend; Fig. 3), compared with 2.15% among those with normal sleep duration group (11.58–13.73%, p < 0.001 for trend) and 2.3% among those with long sleep duration (11.76–14.06%, p < 0.001 for trend).
Fig. 3.
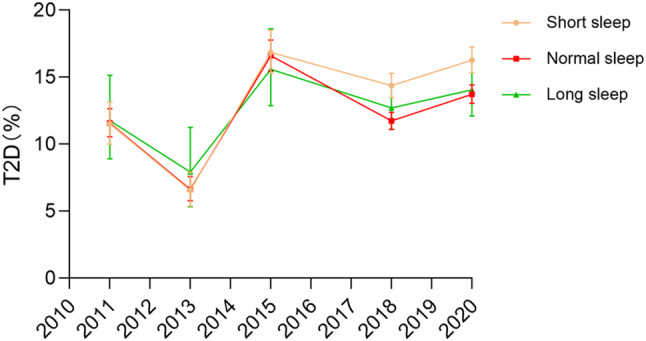
Variation in T2D prevalence over the years across different sleep durations
Independent association of PA and sleep duration with T2D in 2020
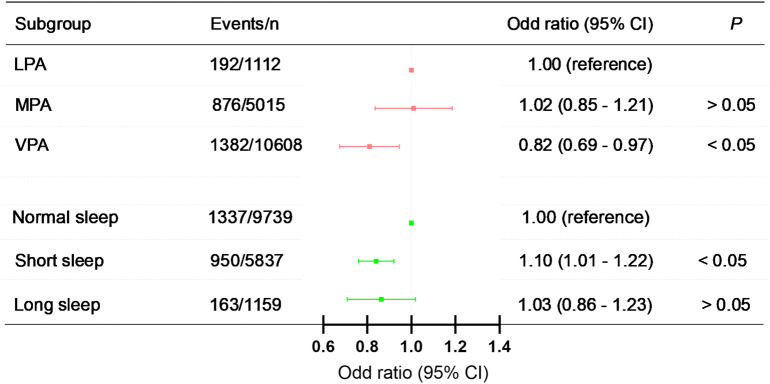
We constructed three multivariable logistic regression models to explore the independent associations. After full adjustment in Model 3, participants with VPA had a lower OR for T2D (Model 3, OR = 0.82, 95% CI = 0.69–0.97; Fig. 4). In contrast, no statistically significant association was observed between MPA and LPA across all models (Model 3, OR = 1.02, 95% CI = 0.85–1.21). Short sleep duration was associated with 10% higher odds of developing T2D (Model 3, OR = 1.10, 95% CI = 1.01–1.22; Fig. 4) when compared to normal sleep duration, whereas long sleep duration was not significantly associated with increased odds of developing T2D (Model 3, OR = 1.03, 95% CI = 0.86–1.23). The results of the other models are presented in Table 4.
Fig. 4.
Independent association of PA, sleep duration and T2D. Adjusted for age, gender, marital status, education level, smoking status, alcohol consumption, place of residence, retirement status and family size as well as hypertension, cancer, stomach disease, liver disease, kidney disease. 95% CI = 95% confidence interval; LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity
Table 4.
Independent associations between PA, sleep duration, and T2D
| Events/n | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|---|
| PA volume | ||||
| LPA | 192/1112 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| MPA | 876/5015 |
1.07 (0.90–1.27) p > 0.05 |
1.00 (0.84–1.19) p > 0.05 |
1.02 (0.85–1.21) p > 0.05 |
| VPA | 1382/10,608 |
0.79 (0.67–0.93) p < 0.05 |
0.80 (0.68–0.95) p < 0.05 |
0.82 (0.69–0.97) p < 0.05 |
| P for trend | p < 0.001 | p < 0.001 | p < 0.01 | |
| Sleep duration | ||||
| Normal | 1337/9739 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Short | 950/5837 |
1.15 (1.05–1.26) p < 0.01 |
1.19(1.09–1.30) p < 0.001 |
1.10 (1.01–1.22) p < 0.05 |
| Long | 163/1159 |
0.93 (0.78–1.12) p > 0.05 |
1.02 (0.85–1.22) p > 0.05 |
1.03 (0.86–1.23) p > 0.05 |
| P for trend | p < 0.05 | p < 0.05 | p > 0.05 | |
Note Model 1 was adjusted for age and gender. Model 2 was adjusted for Model 1 plus marital status, education level, smoking status, alcohol consumption, place of residence, retirement status and family size. Model 3 was adjusted for Model 2 plus hypertension, cancer, stomach diseases, liver diseases, and kidney diseases
Abbreviations 95% CI = 95% confidence interval; LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity; OR = odd ratio; PA = physical activity
Joint associations of PA and sleep duration with T2D in 2020
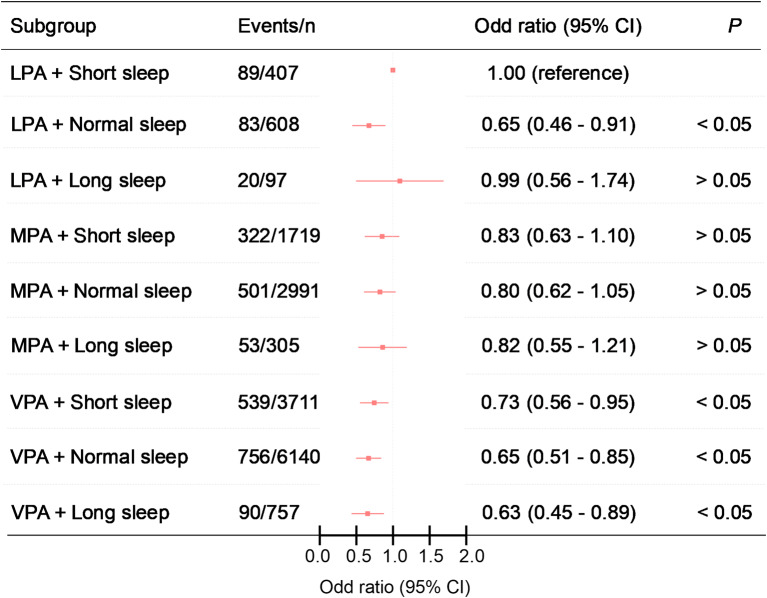
Figure 5; Table 5 present the results of the joint analyses of the association of sleep duration and PA with T2D. Compared to the combination of short sleep duration and LPA, individuals with normal sleep duration who engaged in LPA had lower odds of developing T2D (Model 3, OR = 0.65, 95% CI = 0.46–0.91). For MPA, there was no significant association with T2D when combined with any sleep duration (Model 3, short: OR = 0.83, 95% CI = 0.63–1.10; normal: OR = 0.80, 95% CI = 0.62–1.05; long: OR = 0.82, 95% CI = 0.55–1.21). In contrast, VPA consistently demonstrated lower odds when combined with any sleep duration (Model 3, short: OR = 0.73, 95% CI = 0.56–0.95; normal: OR = 0.65, 95% CI = 0.51–0.85). This protective effect was especially pronounced among those with long sleep durations (OR = 0.63, 95% CI = 0.45–0.89). None of the multiplicative or additive interactions was statistically significant (Supplementary Table S3).
Fig. 5.
Joint association of PA, sleep duration and T2D. Adjusted for age, gender, marital status, education level, smoking status, alcohol consumption, place of residence, retirement status and family size as well as hypertension, cancer, stomach disease, liver disease, kidney disease. 95% CI = 95% confidence interval; LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity
Table 5.
Joint associations between PA, sleep duration and T2D
| Events/n | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|---|
| LPA | ||||
| Short sleep duration | 89/407 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Normal sleep duration | 83/608 |
0.61 (0.44–0.84) p < 0.05 |
0.59 (0.42–0 0.82) p < 0.05 |
0.65 (0.46–0.91) p < 0.05 |
| Long sleep duration | 20/97 |
0.89 (0.51–1.53) p > 0.05 |
0.93 (0.53–1.60) p > 0.05 |
0.99 (0.56–1.74) p > 0.05 |
| MPA | ||||
| Short sleep duration | 322/1719 |
0.87 (0.67–1.14) p > 0.05 |
0.82 (0.62–1.07) p > 0.05 |
0.83 (0.63–1.10) p > 0.05 |
| Normal sleep duration | 501/2991 |
0.80 (0.62–1.03) p > 0.05 |
0.73 (0.56–0.94) p < 0.05 |
0.80 (0.62–1.05) p > 0.05 |
| Long sleep duration | 53/305 |
0.76 (0.52–1.11) p > 0.05 |
0.75 (0.51–1.09) p > 0.05 |
0.82 (0.55–1.21) p > 0.05 |
| VPA | ||||
| Short sleep duration | 539/3711 |
0.67 (0.52–0.86) p < 0.05 |
0.69 (0.53–0.88) p < 0.05 |
0.73 (0.56–0.95) p < 0.05 |
| Normal sleep duration | 753/6140 |
0.58 (0.45–0.74) p < 0.001 |
0.57 (0.44–0.74) p < 0.001 |
0.65 (0.51–0.85) p < 0.05 |
| Long sleep duration | 90/757 |
0.52 (0.37–0.72) p < 0.001 |
0.55 (0.40–0.76) p < 0.001 |
0.63 (0.45–0.89) p < 0.05 |
Note Model 1 was adjusted for age and gender. Model 2 was adjusted for Model 1 plus marital status, education level, smoking status, alcohol consumption, place of residence, retirement status and family size. Model 3 was adjusted for Model 2 plus hypertension, cancer, stomach diseases, liver diseases, and kidney diseases
Abbreviations 95% CI = 95% confidence interval; LPA = light-volume physical activity; MPA = moderate-volume physical activity; VPA = vigorous-volume physical activity; OR = odd ratio; PA = physical activity
Discussion
This study analyzes the association between PA volumes, sleep duration, and T2D among middle-aged and older adults in China, revealing two key findings. First, VPA and a 6–8 h sleep duration were independently associated with lower odds of developing T2D, whereas short sleep duration (< 6 h) was associated with higher odds of T2D. Second, the findings indicated a joint association between PA and sleep duration with T2D, suggesting that combinations of PA and sleep duration interact to influence T2D. Specifically, the VPA group generally had the lowest odds of developing T2D regardless of sleep duration; however, those with LPA and normal sleep duration also had reduced odds of T2D. Overall, these findings indicate the important combined role of PA and sleep duration in the development of T2D.
Several studies have investigated the relationship between PA and T2D, with most findings supporting a negative association between MVPA and T2D [20, 29, 30]. Additionally, some studies have suggested a linear relationship where increasing PA volume is associated with a progressively lower risk of T2D, with no threshold effect [31]. In contrast to previous findings, this study found that only VPA was associated with T2D, whereas MPA was not. One potential reason for this discrepancy could be the methodological differences: our study used self-reported PA data, whereas previous studies employed device-measured assessments. Moreover, it is possible that our study population had unique characteristics that may have influenced the relationship between PA and T2D. Additionally, our focus on VPA highlights the importance of higher-volume PA, while the role of MPA may be less pronounced in this specific context.
The current findings replicate the widely recognized positive association between short sleep duration and T2D based on self-reports [10, 32]. However, this study found no significant association between long sleep duration and increased odds of T2D. These inconsistent findings may be attributed to potential biases in self-reported sleep data, as participants may have overestimated their actual sleep duration, thus influencing the study outcomes. Prolonged sleep duration is typically positively associated with an increased risk of T2D [33]. Prolonged sleep can disrupt the body’s circadian rhythm and metabolic processes, thereby affecting insulin sensitivity and glucose homeostasis [34]. Furthermore, prolonged sleep is often accompanied by extended bed rest, sedentary behavior, and sleep fragmentation, all of which are behavioral risk factors for the development of T2D [35, 36]. This finding suggests that the measurement of sleep duration should rely more on objective assessments.
Recognizing that extending sleep duration may not be a feasible goal for a significant proportion of individuals with short sleep duration, it is essential to explore alternative strategies to mitigate the risk of T2D among this population. Findings from a clinical trial have suggested that engaging in high-intensity exercise may help mitigate impaired blood glucose control following short sleep [37]. Consistent with these findings, an analysis of data from the UK Biobank revealed that individuals with short sleep duration were less likely to develop T2D when they regularly engaged in PA [38]. These complex associations between PA and sleep likely reflect the independent and joint mechanisms through which these factors influence T2D risk. Higher PA may enhance β-cell function [39] and improve insulin sensitivity [40], reducing the risk of developing T2D. Conversely, short sleep duration increases the risk of diabetes through multiple pathways, including impaired insulin sensitivity, alterations in hormonal and neuroendocrine functions, and heightened inflammatory responses [10]. However, PA may also influence sleep and vice versa. These interactions underscore the importance of examining PA and sleep in tandem.
Our study also demonstrated that PA and sleep duration interact in nuanced ways. Short sleep duration was associated with higher odds of developing T2D only when combined with physical inactivity. Furthermore, the VPA group generally had the lowest odds of developing T2D regardless of sleep duration; however, those with LPA and normal sleep duration also showed lower odds. These results suggest that higher PA volume can protect short-sleepers from developing T2D. Additionally, the observed interaction between sleep duration and PA aligns with findings from other studies, suggesting that those with higher levels of PA have a lower risk of developing diabetes or insulin resistance compared to their age-matched counterparts [41]. A small-sample experimental study (n = 10) found that PA partially protected sleep-deprived men from a decline in insulin sensitivity [42]. Our study provides direct and robust evidence supporting the hypothesis that achieving high PA levels may help reduce the heightened risk of T2D in individuals with short sleep duration.
This study underscores the importance of VPA in addressing the higher odds of T2D associated with lack of sleep. First, maintaining a high PA volume is particularly beneficial for individuals experiencing sleep deprivation. Second, future randomized controlled trials targeting individuals with T2D should consider lifestyle interventions based on basic characteristics or by combining increased PA and adjusted sleep duration to elucidate the causal relationships between PA, sleep, and T2D. Third, our findings provide new evidence supporting the notion that increasing PA volume can lower the odds of developing T2D in individuals with short sleep duration.
Strengths and limitations
This study has several notable strengths. The large sample size of 16,735 participants and the detailed epidemiological profile allowed for high-quality data analysis and accurate estimation of the exposure-disease relationship. Furthermore, the study not only focused on sleep duration but also distinguished between different volumes of PA (such as light, moderate, and vigorous), providing more detailed and specific health recommendations. However, our study had some limitations. First, sleep duration and PA volume were primarily obtained through self-reported questionnaires, which may have introduced recall bias or social desirability bias, potentially affecting data accuracy. Although we used a dichotomized PA measure, our assumption that PA volumes were of equal length is unlikely to have influenced the results. Second, as with all observational studies, residual confounding from unmeasured or uncontrolled confounders may persist despite adjustment for known potential confounders. Third, our study could not ascertain causal relationships between PA, sleep duration, and T2D but only determined an association between them. Fourth, the current analyses do not include sedentary behavior, which is an important issue closely related to type 2 diabetes, because the original database did not collect specific information on sedentary behavior. Finally, as the PA volume was presented in interval form, the optimal dose of PA matched to sleep duration could not be determined.
Conclusions
Our findings suggest that VPA and sleep durations of 6–8 h are independently associated with lower odds of developing T2D, whereas short sleep duration (less than 6 h) is associated with higher odds of developing T2D. In addition, this study highlighted the joint association between PA and sleep duration in relation to T2D. Specifically, individuals engaging in VPA generally exhibited the lowest odds of developing T2D, irrespective of sleep duration, and those engaging in LPA coupled with normal sleep duration also showed reduced odds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was conducted using data from the China Health and Retirement Longitudinal Study (CHARLS). We extend our gratitude to all the volunteers and staff who participated in this research.
Author contributions
Xuewen Tian conceived and supervised the study. Xuewen Tian and Zigui Zhou designed the experiments. Zigui Zhou analyzed the data. Zigui Zhou wrote the manuscript. All authors approved the final version of the submitted manuscript.
Funding
The study was conducted without any external funding support.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation. Further inquiries can be directed to the first author. The link to my data is “https://figshare.com/s/0f98434ed228dcdec2b9”.
Declarations
Ethics approval and consent to participate
Ethical approval for the CHARLS was obtained from the institutional review board at Peking University. Each respondent who agreed to participate in the survey provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the global burden of Disease Study 2021. Lancet. 2024;403(10440):2204–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Lancet. Diabetes: a defining disease of the 21st century. Lancet. 2023;401(10394):2087. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 Randomized Clinical Trial. JAMA. 2019;321(9):880–90. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 201;14(2):88–98. [DOI] [PubMed]
- 8.Larcher S, Benhamou PY, Pépin JL, et al. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–71. [DOI] [PubMed] [Google Scholar]
- 9.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. [DOI] [PubMed] [Google Scholar]
- 10.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–37. [DOI] [PubMed] [Google Scholar]
- 11.Gallardo-Gómez D, Salazar-Martínez E, Alfonso-Rosa RM, et al. Optimal dose and type of physical activity to improve glycemic control inpPeople diagnosed with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2024;47(2):295–303. [DOI] [PubMed] [Google Scholar]
- 12.Cao Z, Min J, Chen H, et al. Accelerometer-derived physical activity and mortality in individuals with type 2 diabetes. Nat Commun. 2024;15(1):5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl1):S77–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Zhuang X, Huang R, et al. Physical activity and weight loss among adults with type 2 diabetes and overweight or obesity: a post hoc analysis of the look ahead trial. JAMA Netw Open. 2024;7(2):e240219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med. 2008;38(10):807–24. [DOI] [PubMed] [Google Scholar]
- 16.Chennaoui M, Arnal PJ, Sauvet F, et al. Sleep and exercise: a reciprocal issue? Sleep Med Rev. 2015;20:59–72. [DOI] [PubMed] [Google Scholar]
- 17.Huang BH, Duncan MJ, Cistulli PA. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. 2022;56(13):718–24. [DOI] [PubMed] [Google Scholar]
- 18.Kredlow MA, Capozzoli MC, Hearon BA, et al. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–49. [DOI] [PubMed] [Google Scholar]
- 19.Youngstedt SD. .Effects of exercise on sleep. Sports Med. 2005;24:355–65. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Chen Y, Feng H, et al. Association of accelerometer-measured sleep duration and different intensities of physical activity with incident type 2 diabetes in a population-based cohort study. J Sport Health Sci. 2024;13(2):222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strain T, Flaxman S, Guthold R, Country Data Author Group, et al. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: a pooled analysis of 507 population-based surveys with 5·7 million participants [J]. Lancet Glob Health. 2024;12(8):e1232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willoughby AR, Alikhani I, Karsikas M, et al. Country differences in nocturnal sleep variability: observations from a large-scale, long-term sleep wearable study [J]. Sleep Med. 2023;110:155–65. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Hu Y, Smith JP, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Zhang W, Zhang W, et al. Level of physical activity among middle-aged and older Chinese people: evidence from the China health and retirement longitudinal study. BMC Public Health. 2020;20(1):1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The IPAQ group. scoring protocol [EB/OL]. https://docs.google.com/viewer? A = v&pid = sites&srcid = ZGVmYXVsdGRvbWFpbnx0aGVpcGFxfGd4OjE0NDgxMDk3NDU1YWRlZTM. Accesed 20 Oct 2018.
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, Jin X, Shan Z, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9):e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consensus Conference Panel, Watson NF, Badr MS et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep. 2015;38(8):1161-83. [DOI] [PMC free article] [PubMed]
- 29.American Diabetes Association Professional Practice Committee. 3. Prevention or delay of type 2 diabetes and associated comorbidities: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S39-S45. [DOI] [PubMed]
- 30.Aune D, Norat T, Leitzmann M, et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–42. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Zhang W, Xing Z. Device-measured physical activity and type 2 diabetes mellitus risk. Front Endocrinol (Lausanne). 2023;14:1275182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Chen G, Wen J, et al. Association between sleep duration and incidence of type 2 diabetes in China: the REACTION study. Chin Med J (Engl). 2022;135(10):1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han H, Wang Y, Li T, et al. Sleep duration and risks of incident cardiovascular disease and mortality among people with type 2 diabetes. Diabetes Care. 2023;46(1):101–10. [DOI] [PubMed] [Google Scholar]
- 34.Jang JH, Kim W, Moon JS, et al. Association between sleep duration and incident diabetes mellitus in healthy subjects: a 14-year longitudinal cohort study. J Clin Med. 2023;12(8):2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokhlesi B, Temple KA, Tjaden, et al. Association of self-reported sleep and circadian measures with glycemia in adults with prediabetes or recently diagnosed untreated type 2 diabetes. Diabetes Care. 2019;42(7):1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappuccio FP, D’Elia L, Strazzullo P, et al. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saner NJ, Lee MJ, Kuang J, et al. Exercise mitigates sleep-loss-induced changes in glucose tolerance, mitochondrial function, sarcoplasmic protein synthesis, and diurnal rhythms. Mol Metab. 2021;43:101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koloverou E, Esposito K, Giugliano D, et al. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014;63(7):903–11. [DOI] [PubMed] [Google Scholar]
- 39.Heiskanen MA, Motiani KK, Mari A, et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance:a randomised controlled trial. Diabetologia. 2018;61(8):1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirwan JP, Solomon TPW, ojta DM, et al. Effects of 7days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297(1):E151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuo H, Shi Z, Yuan B, et al. Interaction between physical activity and sleep duration in relation to insulin resistance among non-diabetic Chinese adults. BMC Public Health. 2012;12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanHelder T, Symons JD, Radomski MW. Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med. 1993;64(6):487–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation. Further inquiries can be directed to the first author. The link to my data is “https://figshare.com/s/0f98434ed228dcdec2b9”.