Abstract
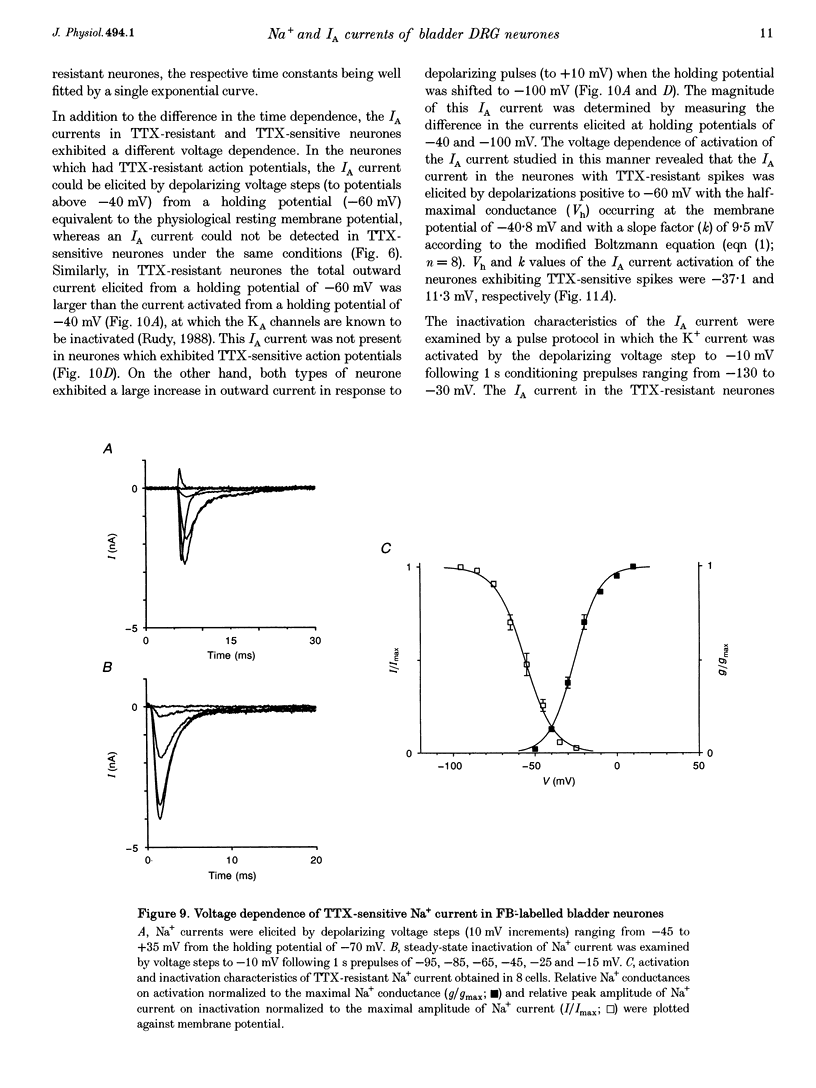
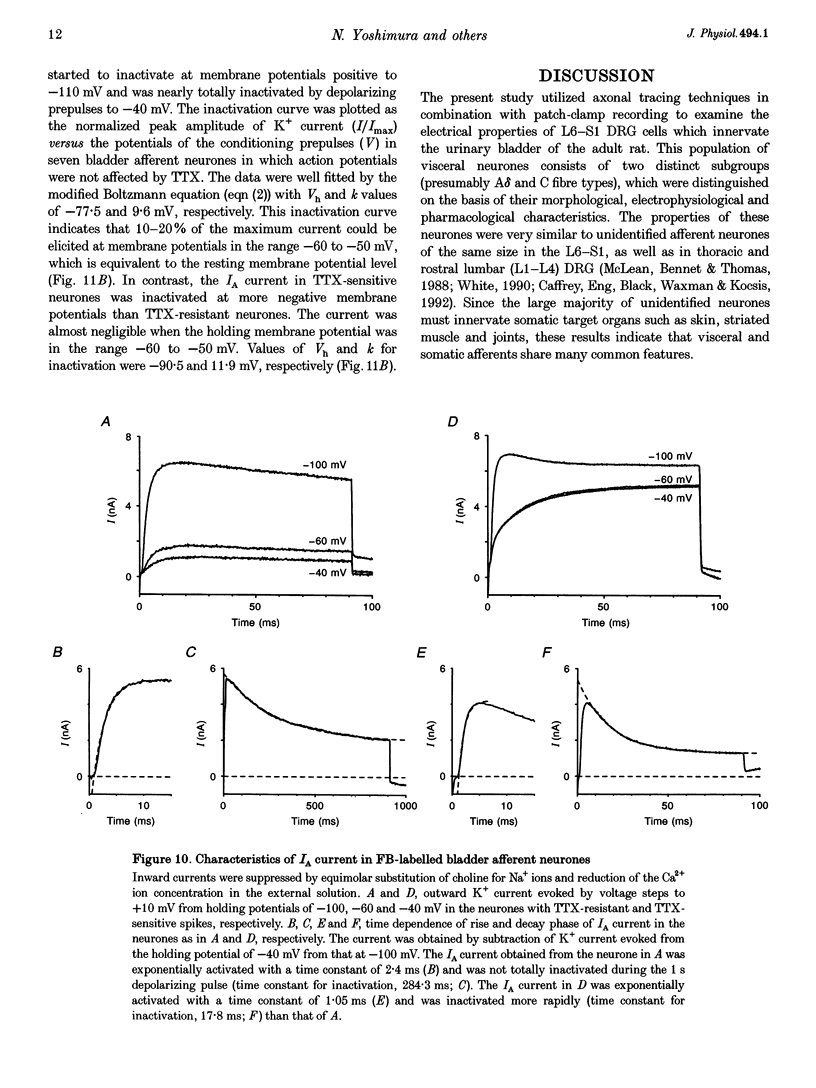
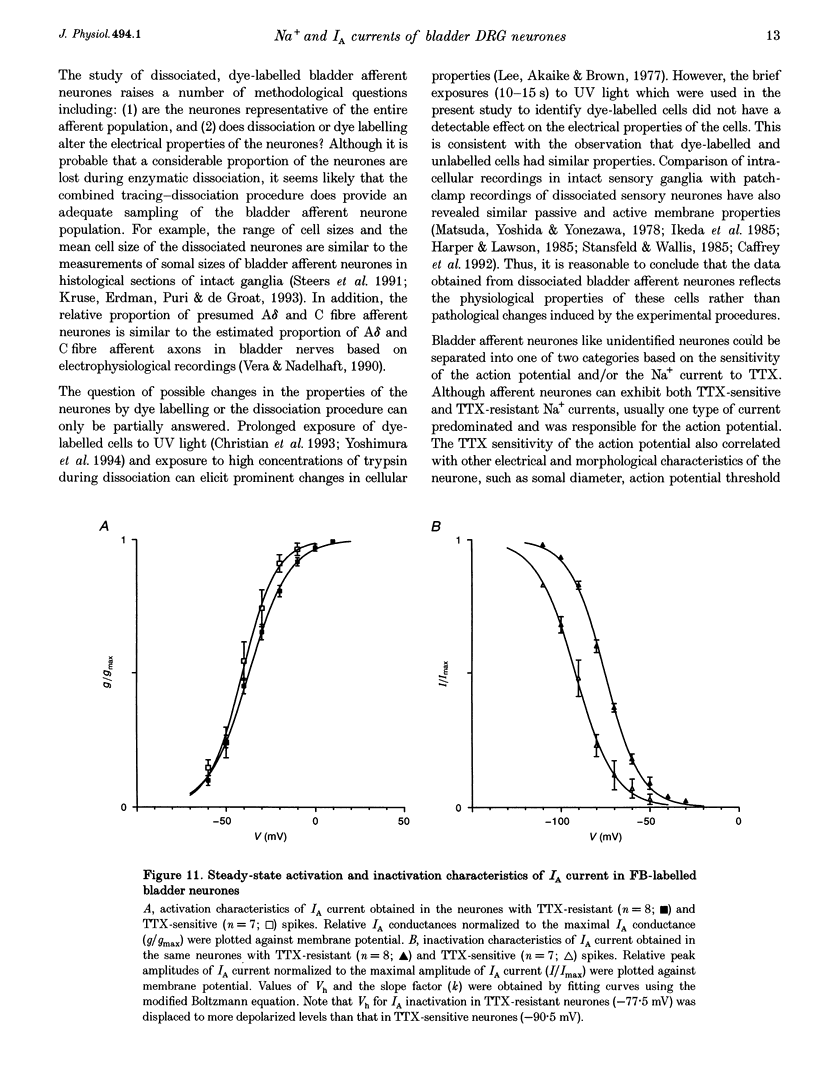
1. Whole-cell patch-clamp recording in combination with axonal tracing techniques was used to examine the electrical properties of afferent neurones innervating the urinary bladder of the adult rat. Individual bladder afferent cells were labelled by Fast Blue (FB), injected into the bladder wall. 2. Passive and active electrical parameters at room temperature (20-22 degrees C) in FB-labelled bladder afferent neurones were comparable with those in unlabelled neurones. Unselected dorsal root ganglion (DRG) neurones as well as bladder afferent neurones exhibited two different types of action potential: high-threshold humped spikes in small-sized neurones and low-threshold narrow spikes in large-sized neurones. 3. The majority (70%) of bladder neurones which were small in size expressed high-threshold tetrodotoxin (TTX)-resistant Na+ channels and slow-inactivating A-type K+ channels (KA), which were available at the resting membrane potential, whereas large-sized DRG neurones had low-threshold TTX-sensitive Na+ channels and fast-inactivating KA channels, which were almost completely inactivated at the resting membrane potential. 4. Half-maximal conductances of activation of TTX-resistant and TTX-sensitive Na+ currents were obtained at -10.3 and -25.3 mV, respectively. The TTX-resistant and TTX-sensitive Na+ currents were half-inactivated at -25.3 and -56 mV, respectively. 5. In the TTX-resistant neurones, the transient outward K+ current (A-type current, IA) with half-maximal conductance at -40.8 mV was half-inactivated at -77.5 mV, and exhibited slower decaying kinetics (mean decay constant (tau), 240 ms) than the IA current recorded from the large-sized TTX-sensitive neurones (mean tau, 20 ms). 6. These results suggest that the majority of bladder afferent neurones have high electrical thresholds for spike activation due to the TTX-resistant Na+ current and the slow-inactivating IA current, which reflect the large population of unmyelinated high-threshold C fibre afferents that innervate the urinary bladder.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Eng D. L., Black J. A., Waxman S. G., Kocsis J. D. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992 Oct 2;592(1-2):283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Ma C. P., de Groat W. C. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995 Apr 24;678(1-2):40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Togo J. A., Naper K. E., Koschorke G., Taylor G. A., Weinreich D. A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. J Neurosci Methods. 1993 Apr;47(1-2):147–160. doi: 10.1016/0165-0270(93)90031-l. [DOI] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. J Gen Physiol. 1989 Nov;94(5):881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993 Apr;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall M., Lindström S., Mazières L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990 Aug;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Leeman S. E., Holzer P., Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman I., Rang H. P. Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells. Neurosci Lett. 1985 May 1;56(1):69–75. doi: 10.1016/0304-3940(85)90442-2. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Hu-Tsai M., Winter J., Woolf C. J. Regional differences in the distribution of capsaicin-sensitive target-identified adult rat dorsal root ganglion neurons. Neurosci Lett. 1992 Aug 31;143(1-2):251–254. doi: 10.1016/0304-3940(92)90276-d. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990 Jun;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Jänig W., Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst. 1990 Jul;30 (Suppl):S89–S96. doi: 10.1016/0165-1838(90)90108-u. [DOI] [PubMed] [Google Scholar]
- Keast J. R., Booth A. M., de Groat W. C. Distribution of neurons in the major pelvic ganglion of the rat which supply the bladder, colon or penis. Cell Tissue Res. 1989 Apr;256(1):105–112. doi: 10.1007/BF00224723. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-III. Potassium currents. Neuroscience. 1981;6(12):2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Kruse M. N., Erdman S. L., Puri G., de Groat W. C. Differences in Fluorogold and wheat germ agglutinin-horseradish peroxidase labelling of bladder afferent neurons. Brain Res. 1993 Jun 11;613(2):352–356. doi: 10.1016/0006-8993(93)90926-e. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Mallory B., Steers W. D., De Groat W. C. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989 Aug;257(2 Pt 2):R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Yoshida S., Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res. 1978 Oct 6;154(1):69–82. doi: 10.1016/0006-8993(78)91052-1. [DOI] [PubMed] [Google Scholar]
- McFarlane S., Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991 Oct;66(4):1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- MeLean M. J., Bennett P. B., Thomas R. M. Subtypes of dorsal root ganglion neurons based on different inward currents as measured by whole-cell voltage clamp. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):95–107. doi: 10.1007/BF00231008. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I., Booth A. M. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984 Jun 20;226(2):238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Kinetic analysis of two types of Na+ channels in rat dorsal root ganglia. J Physiol. 1993 Jul;466:9–37. [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Ontogenic development of the TTX-sensitive and TTX-insensitive Na+ channels in neurons of the rat dorsal root ganglia. Brain Res Dev Brain Res. 1992 Jan 17;65(1):93–100. doi: 10.1016/0165-3806(92)90012-l. [DOI] [PubMed] [Google Scholar]
- Roy M. L., Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992 Jun;12(6):2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Stansfeld C. E., Wallis D. I. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol. 1985 Aug;54(2):245–260. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- Steers W. D., Ciambotti J., Etzel B., Erdman S., de Groat W. C. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991 Aug 15;310(3):401–410. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P. L., Nadelhaft I. Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Res. 1990 Jun 18;520(1-2):83–89. doi: 10.1016/0006-8993(90)91693-b. [DOI] [PubMed] [Google Scholar]
- Waddell P. J., Lawson S. N. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience. 1990;36(3):811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. J Neurophysiol. 1990 Jul;64(1):57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., White G., Weight F. F., de Groat W. C. Patch-clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques. J Auton Nerv Syst. 1994 Sep;49(1):85–92. doi: 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]
- de Groat W. C., Kawatani M., Hisamitsu T., Cheng C. L., Ma C. P., Thor K., Steers W., Roppolo J. R. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990 Jul;30 (Suppl):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]



