Abstract
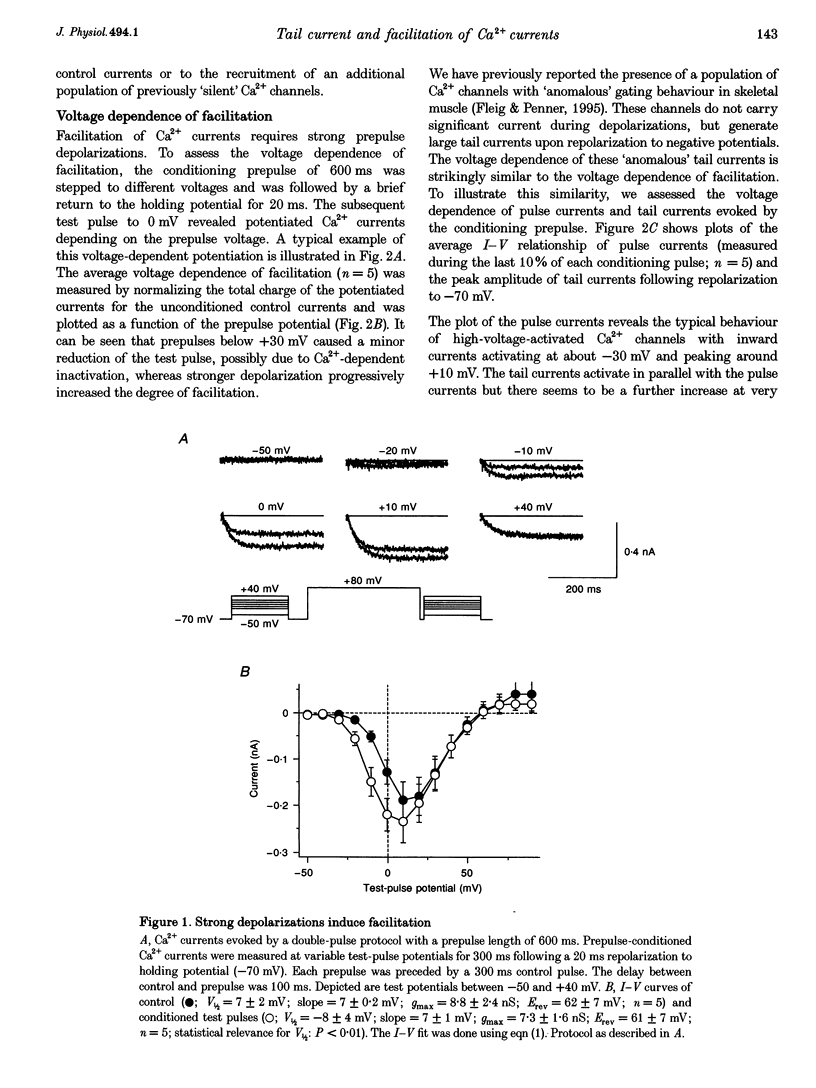
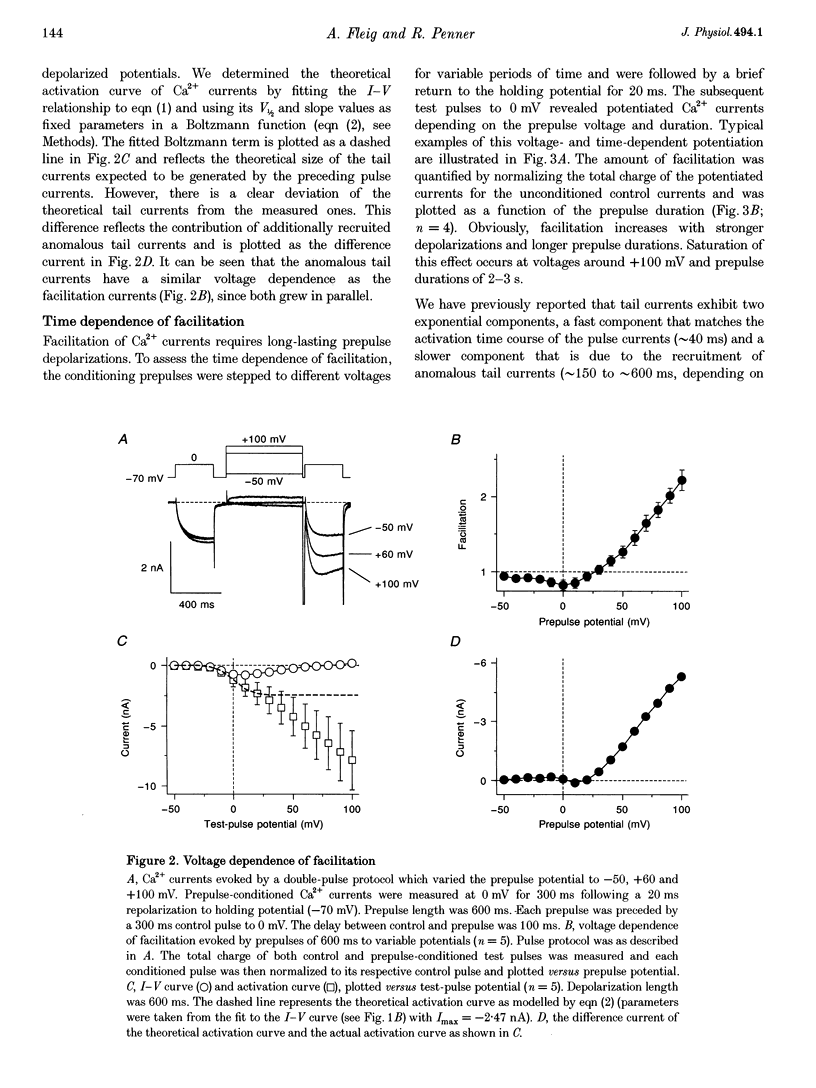
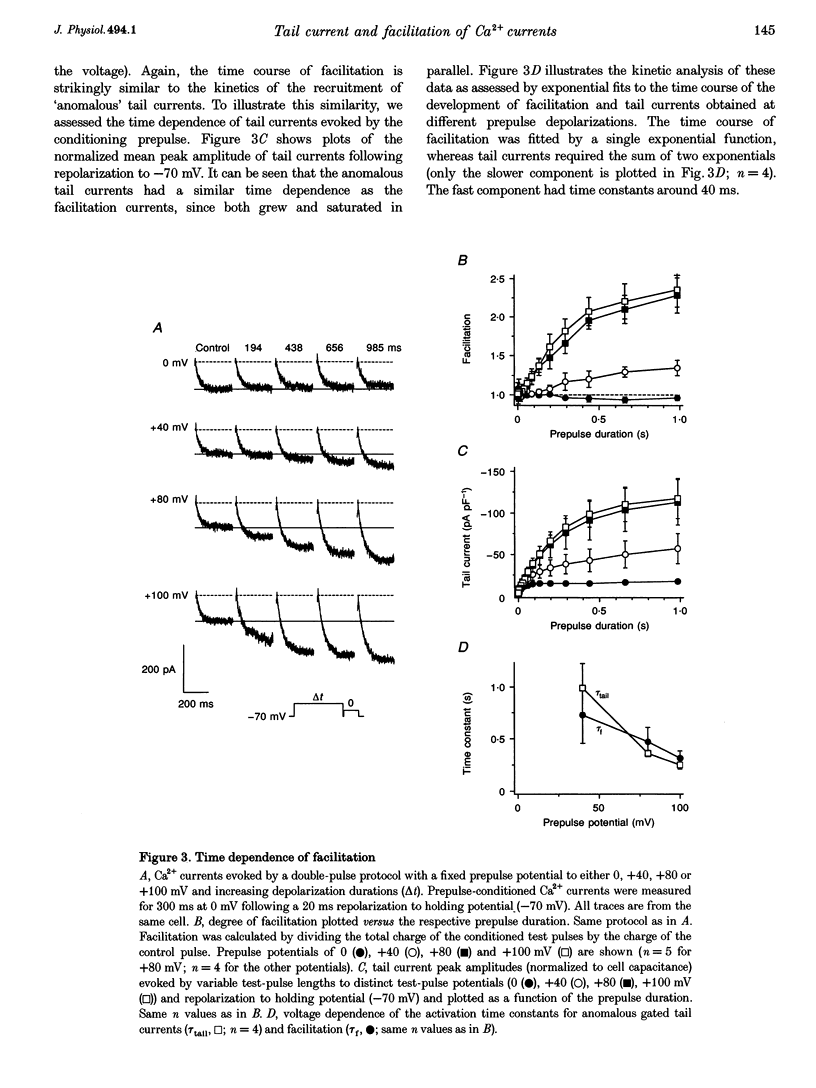
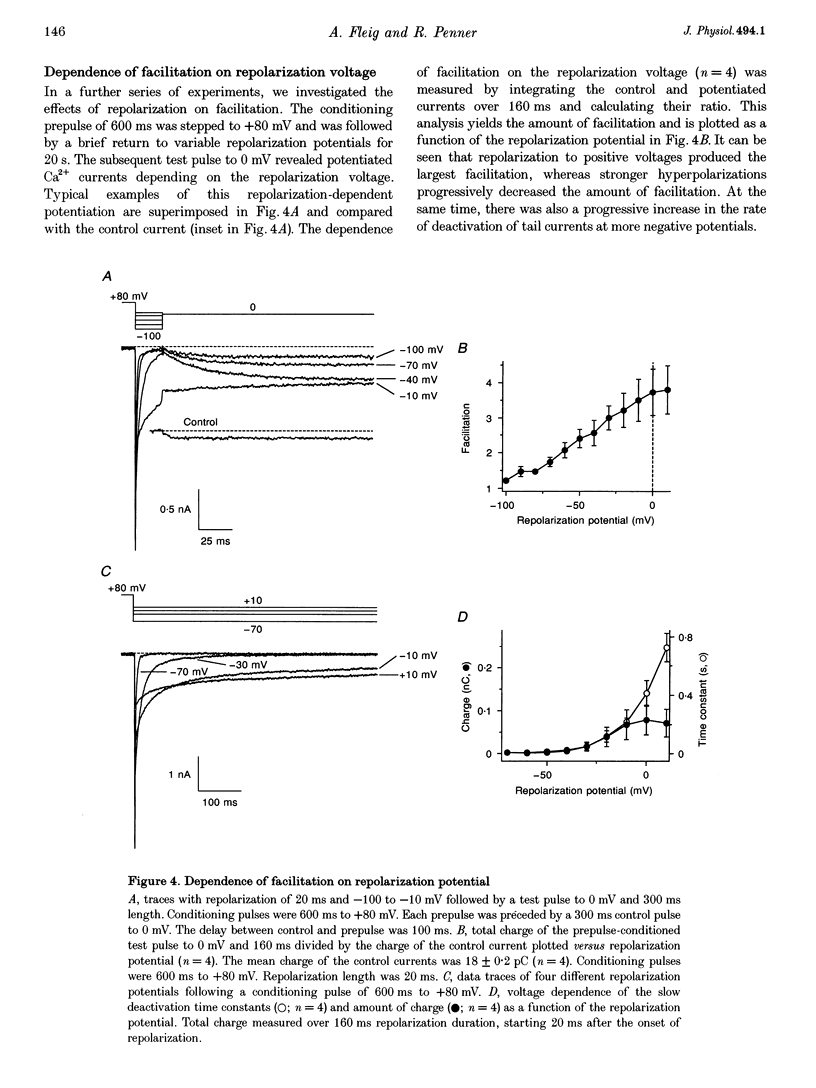
1. Whole-cell patch-clamp recording were employed to study facilitation of Ca2+ currents and excessive Ca2+ tail currents evoked by strong and long-lasting conditioning depolarizations in skeletal myoballs cultured from newborn rats. 2. Paired-pulse facilitation and excessive tail currents showed the same voltage dependence, becoming prominent at conditioning potentials above +30 mV. 3. Recruitment of excessive tail currents and facilitation occurred with the same time dependence (time constant (tau), approximately 200 ms to approximately 1 s), accelerating with the depolarization strength of conditioning pulses. 4. Reversal of Ca2+ current facilitation during the repolarization period between conditioning and test pulses was time- and voltage dependent. The time window of recruitment of facilitated Ca2+ currents narrowed considerably at more negative repolarization potentials (tau: approximately 10 ms at -100 mV, but approximately 1.5 at 0 mV). 5. Neither omission of internal ATP nor perfusion of the cells with the peptide inhibitor of protein kinase A (PKI) had significant effects on Ca2+ current facilitation, although internal perfusion with ATP gamma S slowly suppressed the facilitation currents by about 30%. External application of either ryanodine or caffeine under control conditions selectively and significantly suppressed the facilitated Ca2+ currents by about 30-40%. 6. We propose that facilitation of Ca2+ currents and excessive tail currents are consequences of a common mechanism linked to ryanodine receptors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C. G protein modulation of calcium currents in neurons. Annu Rev Physiol. 1990;52:243–255. doi: 10.1146/annurev.ph.52.030190.001331. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. Fast gating kinetics of the slow Ca2+ current in cut skeletal muscle fibres of the frog. J Physiol. 1990 Jun;425:347–367. doi: 10.1113/jphysiol.1990.sp018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A., Penner R. Excessive repolarization-dependent calcium currents induced by strong depolarizations in rat skeletal myoballs. J Physiol. 1995 Nov 15;489(Pt 1):41–53. doi: 10.1113/jphysiol.1995.sp021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B. E., Andrews S. B., Fleischer S., Marks A. R., Caswell A., Powell J. A. Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J Cell Biol. 1993 Dec;123(5):1161–1174. doi: 10.1083/jcb.123.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Rothlein J., Smith S. J. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. D., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Porzig H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev Physiol Biochem Pharmacol. 1990;114:209–262. doi: 10.1007/BFb0031020. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Activation of a G protein promotes agonist responses to calcium channel ligands. Nature. 1987 Dec 24;330(6150):760–762. doi: 10.1038/330760a0. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993 Jul 15;364(6434):240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Ogunbumni E., Freund E., Wiederrecht G., Marks A. R., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Nov 5;268(31):22992–22999. [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]



