Abstract
The cytosolic factor Cif (cytochrome c-efflux inducing factor) was activated by the apoptosis inducers staurosporine and anti-Fas antibodies and rapidly induced the efflux of cytochrome c from purified human mitochondria. HL-60 cells that stably overexpressed a bcl-2 cDNA transgene (Bcl-2:HL-60 cells) contained mitochondria and a cytosol that were resistant to exogenous Cif and that lacked detectable endogenous Cif activity, respectively. Therefore, Bcl-2 overexpression negated Cif activity and suggested that the requirement for Cif resides upstream of Bcl-2 on the apoptotic signal transduction pathway. The addition of purified caspase 3, caspase 7, or caspase 8 to the cytosolic extract from Bcl-2:HL-60 cells, however, restored Cif activity, demonstrating that the inhibition of Cif by Bcl-2 overexpression could be overcome by activated caspases. Moreover, the addition of purified caspases to cytosolic extracts prepared from parental HL-60 cells was also sufficient to cause Cif activation, suggesting that caspases might be required for Cif activation. Consistent with these observations, Fas-induced apoptosis in Jurkat cells resulted in caspase 8 activation and subsequently in activation of Cif. Finally, we demonstrate that the activation of Cif correlated with the activation of the Bcl-2 family member Bid by caspases and that Cif activity was selectively neutralized by anti-Bid antibodies. Taken together, these results indicate that Cif is identical to Bid and that it can be inhibited by Bcl-2 and activated by caspases. Thus, Cif (Bid) is an important biological regulator for the transduction of apoptotic signals.
Almost all cultured cells can be induced to undergo apoptosis by a plethora of stimuli, including but not limited to DNA damage, exposure to glucocorticords, and withdrawal of growth factors (reviewed in reference 56). Although these stimuli elicit varied initial cellular responses, the final stages of apoptosis are remarkably similar irrespective of the lethal stimulus. In particular, activation of a novel family of cytosolic proteases, termed caspases (1; reviewed in references 8 and 9), is a key common hallmark of apoptotic cells. Caspases are relatively widely and constitutively expressed as inactive zymogens (procaspases), which are themselves proteolytically cleaved to yield an active enzyme (reviewed in references 37 and 48). Caspases can be divided into “initiator” caspases (e.g., caspases 8 and 10), which activate downstream “effector” caspases (e.g., caspases 3, 6, and 7). Effector caspases then cleave a limited subset of cellular proteins (reviewed in reference 44), which appear ultimately to be responsible for the biochemical and morphological changes associated with programmed cell death. The remarkable similarity in the terminal events of apoptosis regardless of the initial apoptotic stimulus strongly implies that there is a common signal transduction pathway leading to activation of caspases in the cytosol.
One of the most extensively studied processes of apoptotic signal transduction is initiated by activated Fas (CD95/Apo-1) (reviewed in reference 36). Fas is a cell membrane receptor which belongs to the tumor necrosis factor receptor superfamily (60). The binding of the Fas ligand (54) or agonistic antibodies against Fas (38) causes the activation of Fas and rapid apoptosis of many cell types (49). Fas contains a motif referred to as the death domain, which is required for apoptosis (21, 55). Biochemically, activated Fas interacts, through its death domain, with the death domain of the FADD/MORT-1 protein (reviewed in reference 7), which in turn recruits procaspase 8, thus forming a death-inducing signaling complex (DISC) (25, 43). Engagement of the Fas receptor results in the oligomerization and activation of caspase 8 (35, 64), which ultimately results in the activation of many downstream effector caspases (4, 33).
Caspase 8 may activate other caspases by directly cleaving them (51), or it may indirectly activate them through the use of the cytochrome c:Apaf-1 signaling pathway (49). Thus, through an unidentified mechanism, caspase 8 activation results in the efflux of cytochrome c, normally an exclusively mitochondrial protein, into the cytosol (reviewed in reference 45). Once in the cytosol, cytochrome c, in the presence of dATP, binds to apoptotic protease-activating factor 1 (Apaf-1) (30), to which is tethered procaspase 9 (29, 42). The binding of cytochrome c to Apaf-1 then facilitates the activation of caspase 9, potentially through an autoactivation process facilitated by Apaf-1-mediated oligomerization (52). Active caspase 9, in turn, activates procaspases 3 and 7 by direct cleavage (29, 52). The activation of caspase 3 in particular is extremely important, since it is the most biologically relevant effector caspase identified to date, being responsible for the cleavage of a large number of target proteins (reviewed in references 9 and 44). The activation of caspase 3 generally also implies that the cell is irretrievably committed to dying. Thus, apoptotic signaling involves a caspase cascade that ultimately results in intracellular proteolysis and death (reviewed in references 37 and 48).
Mitochondrial cytochrome c efflux, with the resultant activation of caspases 9, 3, and 7, appears to be a pivotal control point not only for Fas-mediated apoptosis in certain cell types (49) but also for the chemical induction of apoptosis. This hypothesis is supported by studies which demonstrated that overexpression of the apoptosis suppressor, Bcl-2, in mitochondria of various cells blocked cytochrome c efflux and apoptosis induced by a variety of stimuli (26, 63). Furthermore, the process of cytochrome c efflux during Fas-induced apoptosis in Jurkat cells was blocked by overexpression in mitochondria of the related apoptotic suppressor protein, Bcl-XL (58). Therefore, transduction not only of chemically induced but also of Fas-induced apoptotic signals to mitochondria can be inhibited by overexpression of apoptosis suppressors. Molecular and biochemical studies have demonstrated that apoptotic effectors can physically interact with and inhibit the function(s) of various apoptotic suppressors. In particular, Bad (62) and Bax (40) can inhibit Bcl-2, and Bad (62), Bak (6, 12, 24), and Bax (3) can inhibit Bcl-XL. Since Bax (19, 61) and Bad (65; reviewed in reference 13), which are normally localized in the cytosol, can be actively translocated to mitochondria during apoptosis induction, it is plausible that apoptotic effector proteins such as Bax and/or Bad could serve at the end of the common central apoptotic signal transduction pathway to induce cytochrome c efflux. This hypothesis was supported by a recent study which reported that recombinant Bax proteins directly induced cytochrome c efflux from mitochondria (11, 22). While apoptotic suppressors may have additional functions downstream of cytochrome c efflux (42, 47, 66), it is possible that Bcl-2 and Bcl-XL proteins block the effect of Bax, Bad or other apoptosis effectors on mitochondria and consequently block cytochrome c efflux.
We recently developed a cell-free assay for studying regulation of mitochondrial cytochrome c efflux (17). Using this assay, we observed that treatment of HL-60 cells with the potent apoptosis inducer staurosporine (STS) induced a cytosolic activity, termed Cif (cytochrome c efflux-inducing factor), which rapidly facilitated cytochrome c efflux from purified mitochondria (17). Similar factors and activities have also been described by others (27, 53). Here we demonstrate that the activation of Cif, as well as its action in mitochondria, could be inhibited by Bcl-2 overexpression. This inhibition could be overcome in vitro by the addition of activated caspases. Furthermore, the addition of activated caspases to S-100 extracts prepared from normal cells was sufficient to induce Cif. Thus, Cif activation required an active caspase(s), and its effect could be blocked by apoptotic suppressors. Finally, Cif activity was selectively neutralized by anti-Bid antibodies and the activation of Cif correlated with the cleavage of Bid by caspases. These results suggest that Cif and Bid are one and the same and that it is the biologically relevant regulator of cytochrome c efflux and is thus an important factor in the process of apoptotic signal transduction.
MATERIALS AND METHODS
Materials.
Fetal bovine serum (FBS), EDTA, phenylmethylsulfonyl fluoride (PMSF), aprotinin, antipain, and leupeptin were purchased from Sigma Chemical Co. (St. Louis, Mo.). The Complete protease inhibitor cocktail tablets were purchased from Boehringer Mannheim Co. (Indianapolis, Ind.). Agarose-protein A/G beads were purchased from Oncogene Research Products, Inc. (Cambridge, Mass.). The caspase inhibitor acetyl-DEVD-aldehyde (Ac-DEVD-CHO) was purchased from Quality Controlled Biochemicals, Inc. (Hopkinton, Mass.). Purified active human caspase 3, caspase 7, and caspase 8 and mouse monoclonal antibodies against human caspase 7 and caspase 8 were purchased from Pharmingen, Inc. (San Diego, Calif.). The agonistic antibody against human Fas (clone CH-11) and STS were purchased from Kamiya Biomedical Co. (Seattle, Wash.). Rabbit polyclonal anti-human Bax and Mcl-1 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.), and rabbit polyclonal anti-human Bid antibodies were generously provided by X. Wang (Howard Hughes Medical Institute and Department of Biochemistry, University of Texas Southwestern Medical Center at Dallas, Dallas, Tex.). Mouse monoclonal antibodies against human cytochrome c, Bcl-2, Bax, caspase 2, and caspase 3 have been described previously (17). Protein quantitation reagents were purchased from Bio-Rad Laboratories, Inc. (Hercules, Calif.). Enhanced chemiluminescence Western blot analysis reagents were purchased from Amersham Life Science, Inc. (Arlington Heights, Ill.).
Cells.
The origin of HL-60 and Bcl-2:HL-60 cells used in this study has been described previously (20, 63). The cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1 mM nonessential amino acids, and 1 mM sodium pyruvate. Jurkat cells were cultured in RPMI 1640 medium containing 10% FBS. In addition, all media were supplemented with 100 U of penicillin per ml and 50 U of streptomycin per ml. The cells were incubated at 37°C in a humidified incubator under 5% CO2.
Mitochondrial cytochrome c efflux and caspase 3 activation during apoptosis.
HL-60 cells were left untreated or treated with 5 μM STS for 0, 1, 2, 3, or 4 h. Jurkat cells were suspended in serum-free medium and seeded in culture dishes that had been pretreated with serum-containing (10% FBS) medium for 2 h. To induce apoptosis, anti-Fas antibodies were added to the medium at a final concentration of 150 ng/ml. At the end of each treatment, the cells were pelleted by centrifugation and washed three times in phosphate-buffered saline. The cell pellets were then suspended in 3 volumes of lysis buffer (10 mM HEPES [pH 7.5], 5 mM MgCl2, 1 mM dithiothreitol, 1 mM PMSF, 50 μg each of leupeptin, aprotinin, and antipain per ml) and incubated on ice for 5 min. To lyse the cells, the cell suspension was passed five times through a 26-gauge needle fitted to a syringe. The lysate was divided into two equal volumes. To isolate mitochondria, the lysate was mixed with 12 ml of lysis buffer containing 0.25 M sucrose and the mixture was centrifuged at 500 × g for 10 min at 4°C to pellet the nuclei; the supernatant was then recentrifuged at 10,000 × g for 10 min at 4°C; and the pellet was used as the mitochondrial preparation. The second volume of the initial lysate was immediately centrifuged at 12,500 × g for 5 min at 4°C to pellet the nuclei and mitochondria, and the clarified supernatant was used as the cytosolic fraction. The mitochondrial and cytosolic fractions were mixed with equal volumes of a solution containing 10% glycerol, 5% β-mercaptoethanol, and 2% sodium dodecyl sulfate (SDS), and heated at 100°C for 5 min. Aliquots were then electrophoresed in 10% polyacrylamide gels and blotted onto nitrocellulose filters (15). Cytochrome c and caspases were detected with enhanced chemiluminescence Western blot reagents.
Preparation of S-100 cytosolic extracts.
Cells were washed three times with PBS, and then the cell pellet was resuspended in 2 volumes of lysis buffer and incubated on ice for 5 min. To lyse the cells, the cell suspension was passed five times through a 26-gauge needle fitted to a syringe. The lysate was centrifuged at 12,500 × g for 5 min at 4°C, and the pelleted nuclei and mitochondria were discarded. The supernatant was centrifuged again at 12,500 × g for 10 min at 4°C to remove residual mitochondria and heavy membranes. Subsequently, the supernatant was centrifuged at 100,000 × g for 60 min at 4°C, and the clarified supernatant (S-100) was used as the cytosolic extract. The extract was adjusted to contain 5 μg of protein per μl plus 50 mM KCl, 5 mM EDTA, and 2.5× Complete protease inhibitor cocktail and then stored in small aliquots at −80°C (16).
Assay for Cif activity.
Purified mitochondria (17) were resuspended in washing buffer (10 mM HEPES [pH 7.5], 50 mM KCl, 5 mM EDTA) and incubated on ice for 2 min. Aliquots of the suspension containing mitochondria from approximately 2 × 106 cells were transferred into microcentrifuge tubes and centrifuged at 12,500 × g for 1 min to pellet mitochondria. The supernatant was discarded, and the mitochondria were incubated in 20 μl of basic buffer (10 mM HEPES [pH 7.5], 1 mM DTT, 1 mM PMSF, 50 μg each of antipain, aprotinin, and leupeptin per ml, 5 mM MgCl2, 5 mM EDTA, 2.5× Complete protease inhibitor cocktail, 50 mM KCl) or 20 μl of the S-100 extract at 37°C for 15 min. To terminate the incubation, the tubes were centrifuged at 12,500 × g for 1 min. The supernatant was mixed with 20 μl of 2× SDS sample buffer, and the pellet was dissolved in 40 μl of 1× SDS sample buffer. Both samples were heated at 100°C for 5 min, and then 10 μl of each sample was subjected to electrophoresis in a 10% polyacrylamide gel and subsequently analyzed by immunoblot analysis for the presence of cytochrome c (17). Cif activity was indicated by a reduction of cytochrome c content in the pellet fraction and a corresponding increase of cytochrome c content in the supernatant fraction of each sample.
RESULTS
Activation of Cif during apoptosis induction.
Recently, we developed an in vitro cell-free assay to study cytochrome c efflux from purified mitochondria (17). We demonstrated that incubation of mitochondria purified from HL-60 cells with the cytosolic S-100 extract from HL-60 cells which had been treated with the potent apoptotic inducer STS resulted in cytochrome c efflux. In contrast, incubation of mitochondria with a cytosolic S-100 extract from untreated HL-60 cells did not induce cytochrome c efflux (17). This suggested that STS treatment induced activation of a cytochrome c efflux-inducing factor (Cif). Studies by others have subsequently demonstrated that overexpression of Bcl-2 in the mitochondria of HL-60 cells blocked mitochondrial cytochrome c efflux during the process of apoptosis induction by STS as well as other chemicals (63). Taken together, the above studies suggested that if Cif was biologically relevant, its activity should be abrogated by the overexpression of Bcl-2 in the mitochondria of HL-60 cells. The following studies were directed to this and other issues.
Mitochondrial responses to Cif are Bcl-2 dependent.
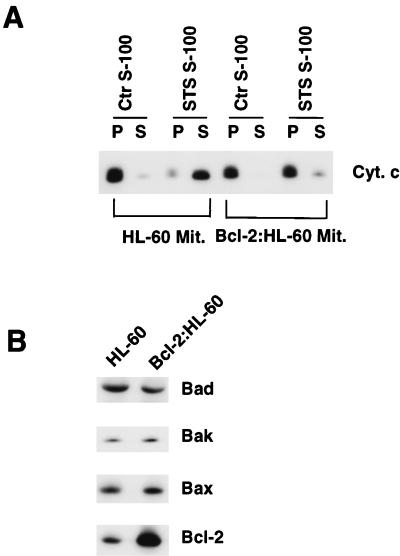
Mitochondria from HL-60 and HL-60 cells that stably overexpressed a bcl-2 cDNA transgene (henceforth referred to as Bcl-2:HL-60 cells [20, 63]) were purified and incubated with 100 μg of S-100 cytosolic extract derived from HL-60 cells that had been treated with 5 μM STS for 60 min (STS S-100), a treatment which was known to induce Cif (17). The STS S-100 extract induced cytochrome c efflux from HL-60 mitochondria but failed to induce significant cytochrome c efflux from Bcl-2:HL-60 mitochondria (Fig. 1A). Western blot analyses of HL-60 and Bcl-2:HL-60 mitochondria showed that whereas the levels of Bad, Bak, and Bax were similar, the level of Bcl-2 was selectively increased by approximately fourfold in Bcl-2:HL-60 mitochondria (Fig. 1B). Therefore, Bcl-2:HL-60 mitochondria were resistant to the effect of Cif, and this was associated with the overexpression of Bcl-2 protein in the mitochondria.
FIG. 1.
Mitochondrial responses to Cif are determined by Bcl-2. (A) HL-60 cells were either left untreated or treated with 5 μM STS for 60 min, and then S-100 cytosolic extract was prepared from the cells. Aliquots of purified HL-60 (HL-60 Mit.) and Bcl-2:HL-60 mitochondria (Bcl-2:HL-60 Mit.) were incubated with 100 μg of the S-100 extract from untreated (Ctr S-100) or STS-treated (STS S-100) HL-60 cells for 15 min at 37°C, and then the samples were centrifuged to pellet the mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c (Cyt. c) content in each fraction was determined by Western blot analysis. A decrease in the cytochrome c content in the P fraction and a corresponding increase in the cytochrome c content the S fraction of a sample indicates that Cif had an effect on the mitochondria. (B) Western blot analyses of Bad, Bak, Bax, and Bcl-2 in mitochondrial extracts from HL-60 and Bcl-2:HL-60 cells. All lanes contained identical amounts of total protein.
Cif activity is not detectable in Bcl-2:HL-60 cells.
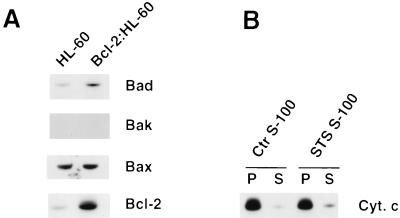
Western blot analyses of S-100 cytosolic extracts from HL-60 and Bcl-2:HL-60 cells revealed that (i) the cytosol of Bcl-2:HL-60 cells contained a slightly elevated level of Bad protein; (ii) Bak protein was not detected in the cytosol of either cell line; (iii) the cytosol of both cells contained similar amounts of Bax; and (iv) Bcl-2 was overexpressed in the cytosol of Bcl-2:HL-60 cells (Fig. 2A). Significantly, the ratio of Bcl-2 to Bax appeared to be approximately 2:1, which strongly suggested that Bcl-2 might exert a dominant effect (reviewed in reference 39) on the cytosolic extract as well as on mitochondria. To experimentally test this hypothesis, Bcl-2:HL-60 cells were treated with 5 μM STS for 60 min and the S-100 cytosolic extract of these cells was incubated with mitochondria purified from normal HL-60 cells and then assayed for the presence or absence of Cif activity. No Cif activity was detected in the STS S-100 extract (Fig. 2B). Thus, the overexpression of Bcl-2 in the cytosol of HL-60 cells also correlated with the absence of Cif activity during induction of apoptosis.
FIG. 2.
Lack of Cif activity in the cytosol of Bcl-2:HL-60 cells. (A) Bcl-2 is overexpressed in the cytosol. Western blot analyses of Bad, Bak, Bax, and Bcl-2 contained in 100 μg of total proteins from HL-60 and Bcl-2:HL-60 S-100 extracts. (B) Bcl-2:HL-60 cells were left untreated or treated with 5 μM STS for 60 min. S-100 cytosolic extracts were then prepared from the cells and incubated with HL-60 mitochondria for 15 min at 37°C. The samples were then centrifuged to pellet the mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c (Cyt. c) content in each fraction was determined by Western blot analysis.
Activation of Fas in Jurkat cells induces Cif activation by a caspase 8-dependent mechanism.
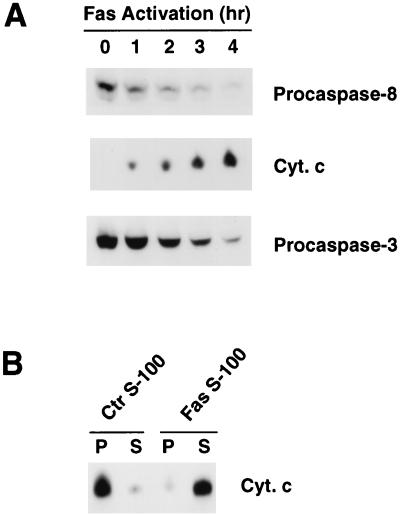
The human Jurkat T-cell leukemia cell line has been extensively used to study apoptosis caused by activation of Fas (reviewed in reference 36). Treatment of Jurkat cells with agonistic antibodies against human Fas induced, in an orderly fashion, activation of caspase 8, accumulation of cytochrome c in the cytosol, and activation of caspase 3 (Fig. 3A). These results suggested that active caspase 8 might be required for mitochondrial cytochrome c efflux. To test this hypothesis, we initially investigated whether Fas activation induced Cif activity. Jurkat cells were left untreated or were treated with agonistic anti-Fas antibodies for 1 h. S-100 cytosolic extracts were then prepared from the cells, and the presence or absence of Cif activity was assayed with purified Jurkat mitochondria. Cif activity was detected in the extract from antibody-treated cells (Fas S-100) but not from untreated cells (Ctr S-100) (Fig. 3B). Thus, Fas activation was capable of inducing Cif activity.
FIG. 3.
Biochemical events following Fas activation in Jurkat cells. (A) Jurkat cells were treated with 150 ng of agonistic antibodies to Fas for 0, 1, 2, 3, and 4 h. At the end of each treatment, cytosolic extract was prepared from the cells and subjected to Western blot analysis for the presence of caspase 8, cytochrome c, and caspase 3. Activation of caspase 8 and caspase 3 was indicated by the reduction in the amounts of the corresponding precursors (procaspases) which remained in the cytosol at the end of each treatment. Mitochondrial cytochrome c efflux was indicated by the presence of cytochrome c (Cyt. c) in the extracts. (B) Jurkat cells were left untreated or treated with 150 ng of agonistic antibodies to Fas per ml for 60 min. S-100 cytosolic extracts were prepared from the cells and then incubated with Jurkat mitochondria for 15 min at 37°C. The samples were then centrifuged to pellet mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c (Cyt. c) content in each fraction was determined by Western blot analysis.
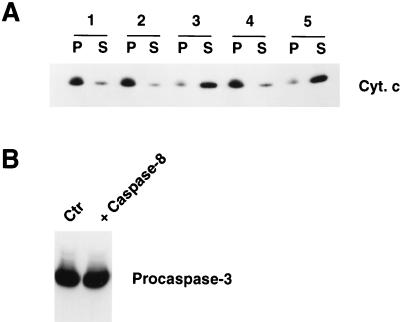
Since Fas activation triggers caspase 8 activation (Fig. 3A), we investigated the possibility that active caspase 8 itself acts as Cif on mitochondria and induces cytochrome c efflux. However, incubation of purified Jurkat mitochondria with active recombinant caspase 8 did not induce cytochrome c efflux (Fig. 4A, panel 2). Therefore, caspase 8 alone could not function as Cif. Incubation at 37°C for 60 min of 100 ng of caspase 8 with 100 μg of S-100 extract from untreated Jurkat cells, however, produced potent Cif activity (panel 3). The ability of caspase 8 to activate Cif was blocked by the presence of 10 μM Ac-DEVD-CHO peptide, a potent inhibitor of many caspase family members (reviewed in reference 48) (panel 4). Therefore, caspase 8 and/or another Ac-DEVD-CHO-inhibitable caspase was sufficient and necessary to generate Cif activity. Finally, we investigated the possibility that active caspase 8 and another cytosolic factor(s) together constituted Cif. S-100 extract (100 μg) from untreated Jurkat cells was incubated with 100 ng of caspase 8 at 37°C for 60 min. Subsequently, 10 μM Ac-DEVD-CHO peptide was added to the sample, which was then used to induce cytochrome c efflux from purified mitochondria. The final sample still contained potent Cif activity (Fig. 4A, panel 5). Therefore, caspase 8 and/or another Ac-DEVD-CHO-inhibitable caspase was not a component of Cif but was required for Cif activation.
FIG. 4.
Caspase 8 can activate Cif in vitro. (A) Aliquots (100 μg) of S-100 cytosolic extract from Jurkat cells were left untreated (lane 1) or treated with 4 μl (100 ng) of caspase 8 (lane 3) or 4 μl (100 ng) of caspase 8 plus 1 μl of Ac-DEVD-CHO (250 μM) (lane 4) at 37°C for 60 min. Then the samples were incubated with mitochondria for 15 min at 37°C to assay for the presence of Cif activity. Alternatively, the S-100 extract was treated with caspase 8 as in lane 3 and then at the end of the treatment, 10 μM Ac-DEVD-CHO was added to the sample, which was then assayed for Cif activity (lane 5). As an additional control, the mitochondria were incubated with just 25 μl of buffer (no S-100 cytosolic extract) containing 100 ng of caspase 8 (lane 2). At the end of each treatment, the samples were centrifuged to pellet the mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c (Cyt. c) content in each fraction was determined by Western blot analysis. (B) Aliquots (100 μg) of S-100 extract from Jurkat cells were left untreated or treated with 100 ng of caspase 8 at 37°C for 60 min. The samples were then subjected to a Western blot analysis for caspase 3.
Active caspase 8 can directly cleave several procaspases including procaspase 3 (34, 41). In addition, caspase 3 is potently inhibited by Ac-DEVD-CHO (reviewed in reference 48). Therefore, it was possible that incubation of the S-100 cytosolic extract from untreated Jurkat cells with active caspase 8 activated caspase 3, which in turn activated Cif. However, the amount of procaspase 3 in the extract before and after caspase 8 treatment was not changed (Fig. 4B). These results demonstrated that (i) the amount of caspase 8 that was sufficient for Cif activation was insufficient for caspase 3 activation and (ii) Cif was not caspase 3.
In vitro activation of Cif by caspases in S-100 extracts from Bcl-2:HL-60 cells.
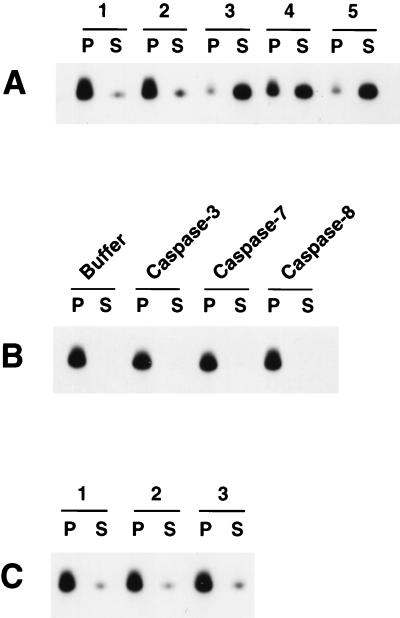
Since caspase 8 could activate Cif in the Jurkat cytosolic extract, we next examined whether purified caspases could overcome the inhibition of Cif caused by Bcl-2 overexpression in Bcl-2:HL-60 cells. S-100 cytosolic extract (100 μg) from Bcl-2:HL-60 cells was either left untreated or incubated with 5 μl of buffer or with 100 ng (5 μl) of active caspase 8, caspase 7, or caspase 3 at 37°C for 60 min. Cif activity was detected in the samples treated with caspase 3 and with caspase 8 and, although to a lesser extent, in the sample treated with caspase 7 (Fig. 5A). Each caspase alone (i.e., without cytosolic extract) had no Cif activity (Fig. 5B). Furthermore, heat treatment (100°C for 10 min) of the caspase-activated samples completely inactivated Cif, such that upon subsequent incubation with purified mitochondria no cytochrome c efflux could be detected (Fig. 5C). From these experiments we concluded that Bcl-2:HL-60 cytosol did indeed still contain Cif, which could be activated by the addition of caspase 8 or caspase 3 and to a lesser extent caspase 7. Furthermore, the activity of caspase-activated Cif was heat sensitive, which was consistent with our previous observations that STS-induced Cif in HL-60 cells was also heat sensitive (17).
FIG. 5.
Cif activation by caspases in the S-100 cytosolic extract from Bcl-2:HL-60 cells. (A) Aliquots (100 μg) of S-100 cytosolic extract from Bcl-2:HL-60 cells were left untreated (lane 1) or treated with 5 μl of buffer alone (lane 2), 5 μl (100 ng) of caspase 3 (lane 3), 5 μl (100 ng) of caspase 7 (lane 4), or 5 μl (100 ng) of caspase 8 (lane 5) for 60 min at 37°C. The samples were then incubated with HL-60 mitochondria for 15 min at 37°C and then centrifuged to pellet the mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c content in each fraction was determined by Western blot analysis. (B) HL-60 mitochondria were incubated with buffer alone or 100 ng of caspase 3, caspase 7, or caspase 8 for 15 min at 37°C and then analyzed as described for panel A. (C) Aliquots (100 μg) of S-100 cytosolic extract from Bcl-2:HL-60 cells were treated with 100 ng of caspase 3 (lane 1), caspase 7 (lane 2), or caspase 8 (lane 3) for 60 min at 37°C. The samples were then heated at 100°C for 10 min, incubated with mitochondria for 15 min at 37°C, and assayed as described for panel A.
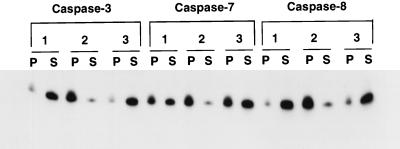
The ability of each of the three caspases to activate Cif in the Bcl-2:HL-60 S-100 extracts was inhibited by Ac-DEVD-CHO (Fig. 6, panels 2). However, once activated by each caspase, Cif activity was no longer inhibitable by Ac-DEVD-CHO (panels 3). This observation was consistent with the effect of caspase 8 on Jurkat cell extract (Fig. 5A) and once again demonstrated that a caspase activity was required for Cif activation but was not itself a component of Cif.
FIG. 6.
Cif activation requires caspases. Aliquots (100 μg) of S-100 cytosolic extract from Bcl-2:HL-60 cells were treated with 100 ng of caspase 3, caspase 7, or caspase 8 in the absence (lanes 1) or presence (lanes 2) of 10 μM Ac-DEVD-CHO at 37°C for 60 min. Alternatively, following caspase treatment, 10 μM Ac-DEVD-CHO was added to the samples (lanes 3). The samples were then incubated with HL-60 mitochondria for 15 min at 37°C and processed as described in the legend to Fig. 5A.
In vitro activation of Cif by caspases does not alter the status of Bcl-2 or Bax proteins in the cytosolic extract.
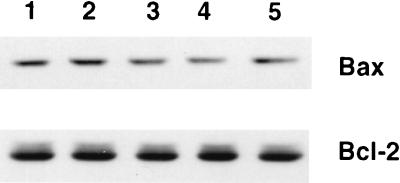
It has been shown that incubation of caspase 3 with Bcl-2 in vitro results in degradation of Bcl-2 (5, 14). Furthermore, caspase 3-processed Bcl-2 protein fragments behave like apoptosis effectors and promote apoptosis (5). Therefore, it was possible that the mechanism by which caspases activated Cif involved Bcl-2 degradation or conversion of Bcl-2 into Cif. Similarly, it was theoretically also possible that caspases processed Bax into Cif. To test these hypotheses, the status of Bcl-2 and Bax was determined by Western blot analyses with 100 μg of S-100 extract derived from Bcl-2:HL-60 cells that was either left untreated (Fig. 7, lane 1), incubated with 5 μl of buffer (lane 2), or incubated with 100 ng (5 μl) of caspase 3, caspase 7, or caspase 8 (lanes 3 to 5, respectively) at 37°C for 60 min. No significant quantitative changes in Bcl-2 or Bax was observed, indicating that the caspase-dependent activation of Cif does not require proteolytic processing of Bcl-2 or Bax (Fig. 7).
FIG. 7.
Absence of Bax and Bcl-2 degradation during caspase-induced Cif activation. Aliquots (100 μg) of S-100 cytosolic extract from Bcl-2:HL-60 cells were left untreated (lane 1) or treated with buffer alone (lane 2) or with 100 ng of caspase 3 (lane 3), caspase 7 (lane 4), or caspase 8 (lane 5) for 60 min at 37°C. The samples were then subjected to immunoblot analyses for Bax and Bcl-2.
Caspase-induced Cif activation correlates with Bid cleavage and its activity is selectively neutralized by Bid antibodies.
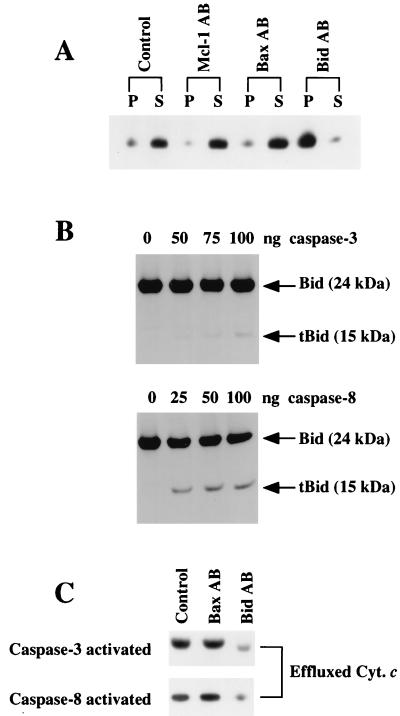
While this paper was being reviewed, two groups reported that the apoptosis effector Bid (59) is cleaved by caspase 8 and that the C-terminal 15-kDa fragment, termed tBid (truncated Bid), was capable of inducing cytochrome c efflux from isolated mitochondria (28, 31). Furthermore, overexpression of Bcl-2 in mitochondria blocked the action of tBid (31). Thus, the activity of tBid appeared similar to the activity of Cif. To experimentally test whether tBid and Cif were identical, we investigated whether Cif activity in the S-100 extract from STS-treated HL-60 cells was sensitive to anti-human Bid antibodies (31). Bid antibodies potently inhibited cytochrome c efflux (Fig. 8A). In contrast, antibodies directed against Mcl-1 and Bax failed to inhibit Cif activity (Fig. 8A). Therefore, Cif activity was selectively neutralized by Bid-specific antibodies. Next, the effect of purified caspase 3 and caspase 8 on endogenous Bid in HL-60 S-100 extracts was determined. Both caspase 3 and caspase 8 cleaved Bid, producing the 15-kDa tBid, although caspase 8 cleaved Bid much more efficiently (Fig. 8B). Furthermore, the Cif activity induced by either caspase 3 (100 ng) or caspase 8 (25 ng) was selectively neutralized by the Bid but not by the Bax antibodies (Fig. 8C). Taken together, these results indicate that tBid is identical to Cif.
FIG. 8.
Cif activity is selectively sensitive to Bid-specific antibodies. (A) A 20-μl volume of S-100 extract from STS-treated HL-60 cells was left untreated (Control) or incubated with 3 μl of rabbit polyclonal antibodies to human Mcl-1 (Mcl-1 AB), Bax (Bax AB), or Bid (Bid AB) at 20°C for 15 min. The extracts were then incubated with HL-60 mitochondria for 15 min at 37°C. The samples were then centrifuged to pellet the mitochondria. The pellet (P) and supernatant (S) were separated, and the cytochrome c content in each fraction was determined by Western blot analysis. (B) Aliquots (100 μg) of S-100 extract prepared from Bcl-2:HL-60 cells were treated with various amounts of caspase 3 or caspase 8 for 60 min at 37°C. The samples were then subjected to electrophoresis and Western blot analyses to determine the status of Bid. (C) Aliquots (100 μg) of S-100 extract prepared from Bcl-2:HL-60 cells were treated with 100 ng of caspase 3 or 25 ng of caspase 8 for 60 min at 37°C. Then the samples were either left untreated (Control) or incubated with 3 μl of rabbit polyclonal anti-human Bax (Bax AB) or Bid (Bid AB) antibodies at 20°C for 15 min. The extracts were incubated with HL-60 mitochondria for 15 min at 37°C, and the samples were centrifuged to pellet mitochondria. Aliquots of the supernatant were subjected to electrophoresis and Western blot analyses for the presence of effluxed cytochrome c (Cyt. c) by caspase 3-activated samples (top) and caspase 8-activated samples (bottom).
Bcl-2 blocks the activation of caspases regardless of whether they require Cif for their activation.
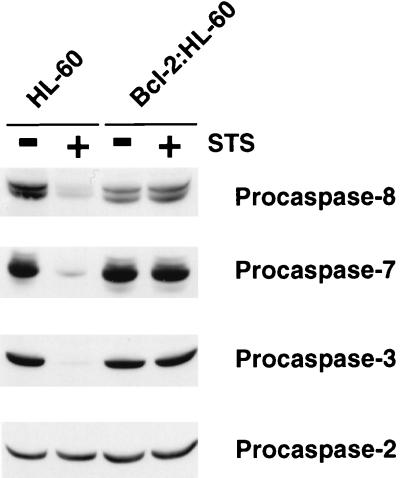
Initiator caspases such as caspase 8 clearly can be activated without cytochrome c efflux (4, 33) whereas effector caspases such as caspase 3, 6, and 7 are activated by a cytochrome c- and thus Cif-dependent mechanism (29, 52). To experimentally test whether Bcl-2 was specifically blocking Cif-dependent caspase activation, we used immunoblot analysis to examine the status of various caspases in HL-60 and Bcl-2:HL-60 cells treated with STS. Caspases 3, 7, and 8 were readily activated by STS of HL-60 cells (Fig. 9). None of these caspases was activated in STS-treated Bcl-2:HL-60 cells, and caspase-2 was not activated in either cell line (Fig. 9). Therefore, overexpression of Bcl-2 had an apparent general inhibitory effect on the activation of caspases, regardless of whether they required Cif or cytochrome c for their activation.
FIG. 9.
Activation of caspases is blocked in Bcl-2:HL-60 cells. HL-60 and Bcl-2:HL-60 cells were left untreated (−) or treated with 5 μM STS for 4 h (+). S-100 extracts were then prepared from the cells, and samples (100 μg) were subjected to Western blot analyses for the indicated caspases.
DISCUSSION
Cif is a cytosolic activity that is required for cytochrome c efflux (17). In this study, we have demonstrated that Cif activity is regulated by caspases and Bcl-2. In particular, Cif activation by STS was blocked by Bcl-2 overexpression in HL-60 cells (Fig. 2). The refractory nature of the Cif activation induced by Bcl-2 appeared to be associated with the inhibition of activation of numerous caspases, which, at least in vitro, were otherwise capable of activating Cif (Fig. 4 to 6). Therefore, the transduction of STS-induced apoptotic signals appeared to involve the activation of caspases, which activated Cif, which then acted on the mitochondria to facilitate cytochrome c efflux (Fig. 10). Together, these results provide strong evidence for the existence of an apoptotic signal transduction pathway, mediated by Cif, from the cell cytosol to the mitochondria, and they elaborate the regulatory roles played by Cif, Bcl-2, and caspases (Fig. 10).
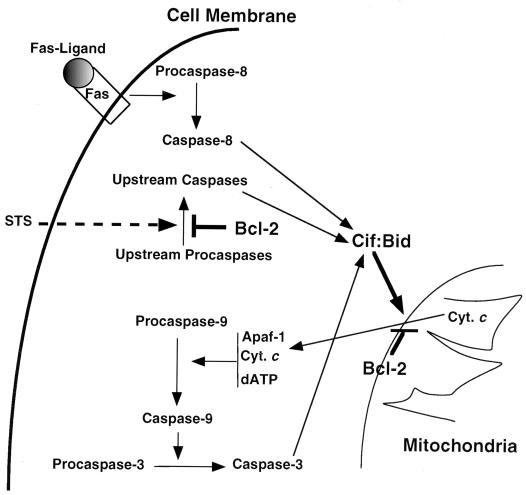
FIG. 10.
Model for the transduction processes of apoptotic signals and their regulation by Bcl-2, caspases, and Cif. The model shows that activation of Fas by its ligand is followed by activation of caspase 8, which subsequently activates Cif/Bid either directly or indirectly by activating other upstream caspases. Alternatively, apoptotic signals generated by STS treatment can lead to the activation of an upstream caspase(s), a process that can be inhibited by the presence of Bcl-2 protein in the cytosol. Activation of Cif/Bid represents the convergence of the various apoptotic signals. Cif/Bid acts on the mitochondria and induces cytochrome c (Cyt. c) efflux. It is anticipated that Bcl-2 antagonizes Cif/Bid activity at a site on the outer membrane of mitochondria. The efflux of cytochrome c into the cytosol then activates a proteolytic cascade, leading to the activation of caspase 3 as long as the concentrations of dATP, Apaf-1, and caspase 9 are sufficient.
Cif is Bid and not Bax.
At the beginning of this study, Bax was considered a likely candidate for Cif, particularly since (i) recombinant Bax was reported to induce cytochrome c efflux (11, 22); (ii) Bax can translocate from the cytosol to the mitochondria during apoptosis (61); (iii) the activity of Bax is antagonized by Bcl-2 (40); (iv) overexpression of Bax in some, but not all, cell types induced apoptotic activities including mitochondrial cytochrome c efflux (reviewed in references 45 and 46); and (v) the cytosol of HL-60 cells contained Bax (Fig. 2A). Therefore, it was plausible that in HL-60 cells cytosolic Bax was Cif, which was translocated to mitochondria and induced cytochrome c efflux during the process of apoptosis. In Bcl-2:HL-60 cells, most, if not all, Bax protein in the cytosol would be neutralized by Bcl-2 and thus cytochrome c efflux would be abrogated. Lastly, treatment of the S-100 cytosolic extract from Bcl-2:HL-60 cells with various caspases, including caspase 3, produced active Cif (Fig. 5 and 6), and it has been shown that treatment of Bcl-2 with caspase 3 resulted in the degradation of Bcl-2 (5, 14). Therefore, it was possible that caspase treatment degraded the excess Bcl-2 protein and freed Bax, which then acted on the mitochondria to induce cytochrome c efflux. This hypothesis, however, seems unlikely. First, Cif-induced cytochrome c efflux from HL-60 mitochondria occurred without any change in the amount of Bax in the mitochondria (17). Second, although treatment of the Bcl-2:HL-60 S-100 cytosolic extract with caspases activated Cif, there was no apparent degradation of Bcl-2 (Fig. 7), indicating that it was unlikely that the caspase treatment was altering the ability of Bcl-2 to inhibit Bax. Lastly, antibodies directed against Bax were incapable of neutralizing caspase 3-, caspase 8-, or STS-activated Cif activity (Fig. 8). Thus, it seems highly likely that Cif is not Bax.
Another candidate for Cif was Bid, since very recent studies by two independent groups using quite different approaches demonstrated that Bid is cleaved by caspase 8 in Fas-activated cells (28, 31). The resulting truncated Bid fragment (tBid), containing the C-terminal 15 kDa of Bid, was responsible for inducing cytochrome c efflux from isolated mitochondria (28, 31). Thus, the regulation of Bid by caspase 8 strongly mimicked the regulation observed for Cif. Indeed, it seems likely that Bid and Cif are identical since (i) the activity of Cif produced in STS-treated HL-60 cells or by caspases in vitro was selectively neutralized by Bid-specific antibodies (Fig. 8A and C) and (ii) the activation of Cif by caspases correlated with the production of tBid (Fig. 8B). Furthermore, Cif activation by STS was blocked by Bcl-2 overexpression in HL-60 cells (Fig. 2). Therefore, the transduction of STS-induced apoptotic signals appeared to involve the activation of caspases, which activated Cif/Bid, which then acted on the mitochondria to facilitate cytochrome c efflux.
What is the mitochondrial receptor for Cif/Bid?
The identification of Bid as Cif immediately poses two questions: how does Cif/Bid bind to the mitochondria, and how does it facilitate cytochrome c efflux? Cif/Bid is a Bcl-2 family member that contains a BH3 (Bcl-2 homology 3) domain (59). Through the use of this domain, Cif/Bid is capable of interacting with other Bcl-2 family members (59; reviewed in reference 46). Therefore, a plausible hypothesis is that Cif/Bid and Bcl-2 compete for binding to the same target protein in the membrane of mitochondria, much in the same way that cytochrome c and Bcl-2/Bcl-XL are thought to compete for binding to Apaf-1 (reviewed in reference 18). The results of Cif/Bid or Bcl-2 binding, however, would have different biological results. Thus, binding of Bcl-2 to the target protein would block, whereas binding of Cif/Bid would facilitate, cytochrome c efflux. Indeed, mitochondria derived from Bcl-2:HL-60 cells were resistant to the effect of Cif/Bid (Fig. 1), indicating that mitochondrial Bcl-2 can antagonize Cif/Bid (Fig. 10). In this regard, mitochondrial Bax and Bad would be strong candidates for the target protein. This model is also attractive since it would explain why the simple overexpression of Bax (40) or Bad (62) in the mitochondria of some cells does not result in spontaneous apoptosis but is still dependent upon apoptosis induction.
Alternatively, Cif/Bid may interact with the mitochondria through a novel domain and utilize its BH3 domain solely to facilitate cytochrome c efflux. The observation that a BH3 mutant of Bid is capable of binding to mitochondria but incapable of inducing cytochrome c efflux favors this model (31). Additional experimentation will be required to identify (i) the mitochondrial receptor for Bid and (ii) the Bcl-2 family member which mediates cytochrome c efflux in a Bid BH3-dependent fashion.
Cif/Bid regulation by caspases.
Activation of Fas by Fas ligand or agonistic anti-Fas antibodies induces the formation of the DISC complex on the cytoplasmic side of the cell membrane, which then activates procaspase 8 (25, 43). Activated caspase 8 is then believed to activate downstream “effector” caspases, which cleave cellular targets and facilitate apoptosis (Fig. 10). In addition, recent studies demonstrated that apoptosis induction by Fas in Jurkat cells involves mitochondrial cytochrome c efflux (49, 58). Furthermore, this process was blocked by treatment of the cells with a general caspase inhibitor, zVAD-fmk, suggesting a role for caspases in the process of mitochondrial cytochrome c efflux (58). Therefore, it was possible that caspase 8 or an effector caspase acts directly on mitochondria and induces cytochrome c efflux. Alternatively, the caspase 8 or effector caspase could activate a factor(s), which targets mitochondria and induces cytochrome c efflux. The former possibility seems unlikely since the incubation of mitochondria with exogenous caspase 8 did not induce cytochrome c efflux (Fig. 4A). Rather, our data indicated that caspase 8 activated a factor (Cif/Bid) which then acted on mitochondria and induced cytochrome c efflux (Fig. 4A). A similar conclusion was drawn in recent studies in which it was demonstrated that caspase 8 was able to activate a Cif-like activity which was responsible for inducing mitochondrial cytochrome c efflux (27, 28, 31). Whether caspase 8 directly activates Cif/Bid in vivo or does so through activation of another caspase is still unclear. If the latter possibility is correct, it seems highly unlikely that the caspase which activates Cif/Bid is caspase 3 in spite of the potent inhibition by Ac-DEVD-CHO (57) and the (albeit weak) ability of purified caspase 3 to cleave Bid in vitro (Fig. 8), since the amount of caspase 8 that was required to activate Cif/Bid was not sufficient to activate caspase 3 (Fig. 4B). This strongly suggests that before active caspase 8 can accumulate to a level sufficiently high for activation of effector caspases such as caspase 3, it can activate Cif/Bid, which then acts on the mitochondria and induces cytochrome c efflux.
Role of apoptosis suppressors.
The Bcl-2 family of apoptosis suppressors is thought to abrogate apoptosis at least in part by blocking cytochrome c efflux (10, 23, 26, 58, 63). Our results clearly support this hypothesis. Thus, purified Bcl-2:HL-60 mitochondria, which contained at least four times the normal amount of Bcl-2, were refractory to cytochrome c efflux when exposed to activated HL-60 cytosol (Fig. 1). The mechanism by which apoptosis suppressors block cytochrome c efflux is unclear. The structures of Bcl-2 and Bcl-XL resemble pore-forming domains of bacterial toxin proteins (32, 50), and it has been suggested that Bcl-2 and Bcl-XL may directly regulate the efflux of cytochrome c and/or may regulate the flow of ions into the mitochondria and indirectly affect cytochrome c efflux (reviewed in references 45 and 46). Alternatively, the proapoptotic protein Bax can also form ion channels in synthetic membranes and these could actively facilitate cytochrome c efflux (2). Since Bcl-2 and Bcl-XL are known to physically interact with and neutralize Bax (40), they may act by suppressing the ion channel activity of Bax. Regardless of the actual mechanism by which the Bcl-2 family suppresses cytochrome c efflux, our data support the notion that overexpression of apoptosis suppressors can block cytochrome c efflux induced by activated Cif/Bid. Similar observations have been made recently by Yuan, Wang and their colleagues (28, 31).
Unexpectedly, it has been demonstrated that overexpression of apoptosis suppressors can also function downstream of cytochrome c efflux to block apoptosis (42, 47, 66). In particular, it has been hypothesized that the additional inhibition caused by Bcl-2 and Bcl-XL may be due to their ability to compete for binding to Apaf-1, the cytosolic cytochrome c receptor (reviewed in reference 18). This hypothesis was supported by the recent demonstration that Bcl-XL can physically interact with Apaf-1 (42). This mechanism clearly cannot explain the apoptotic resistance of Bcl-2:HL-60 cells (63). In particular, the cytosolic extract prepared from Bcl-2:HL-60 cells was unable to induce cytochrome c efflux from normal HL-60 mitochondria (Fig. 2). Since cytochrome c is not even fluxed in this situation, Bcl-2 cannot be inhibiting apoptosis by competing with its binding to Apaf-1. Instead, our data strongly suggest that Bcl-2 is inhibiting apoptosis upstream of cytochrome c efflux from the mitochondria. Specifically, Bcl-2 prevented the activation of Cif/Bid (Fig. 2). We believe that this is due to the ability of Bcl-2 to inhibit an upstream initiator caspase similar or identical to caspase 8. This hypothesis is consistent with the finding that the addition of exogenous activated caspases could overcome the inhibition caused by Bcl-2 (Fig. 5). In summary, previous studies have suggested that the apoptosis suppressor Bcl-2 can inhibit apoptosis by two distinct mechanisms: by competitively binding to Apaf-1 to prevent caspase 9 activation and by blocking cytochrome c efflux. Here, by breaking down the cytochrome c efflux mechanism in vitro into two essential parts (mitochondria and cytosol), we have confirmed that Bcl-2 can block cytochrome c efflux. In addition, we have demonstrated that inhibition can occur by a third mechanism; namely, by abrogating Cif activation, probably due to the inhibition (direct or indirect) of caspase activation.
ACKNOWLEDGMENTS
We thank A.-K. Bielinsky for her careful reading of the manuscript and her helpful comments. We thank X. Wang (Howard Hughes Medical Institute and Department of Biochemistry, University of Texas Southwestern Medical Center at Dallas, Dallas, Tex.) for generously providing us with his Bid antibodies.
This work is supported in part by National Science Foundation grant MCB-9630362 (to J.H.W. and E.A.H.) and by National Institutes of Health grant AI35763 (to E.A.H.). E.A.H. is a Leukemia Society of America Scholar.
REFERENCES
- 1.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J J, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou J-C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 3.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. Bcl-X, a Bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 4.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng E H-Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 6.Chittenden T, Harrington E A, O’Connor R, Flemington C, Lutz R J, Evan G I, Guild B C. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 7.Cleveland J L, Ihle J N. Contenders in FasL/TNF death signaling. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 10.Duckett C S, Li F, Wang Y, Tomaselli K J, Thompson C B, Armstrong R C. Human IAP-like protein regulates programmed cell death downstream of Bcl-XL and cytochrome c. Mol Cell Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou J-C. Bax-induced cytochrome c release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow S N, White J H M, Martinou I, Raven T, Pun K-T, Grinham C J, Martinou J-C, Brown R. Cloning of a Bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 13.Gajewski T F, Thompson C B. Apoptosis meets signal transduction: elimination of a Bad influence. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- 14.Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel M R. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998;17:1268–1278. doi: 10.1093/emboj/17.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Carter T H, Reeves W H, Wyche J H, Hendrickson E A. DNA-dependent protein kinase is a target of a CPP32-like apoptotic protease. J Biol Chem. 1996;271:25035–25040. doi: 10.1074/jbc.271.40.25035. [DOI] [PubMed] [Google Scholar]
- 16.Han Z, Hendrickson E A, Bremner T A, Wyche J H. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem. 1997;272:13432–13436. doi: 10.1074/jbc.272.20.13432. [DOI] [PubMed] [Google Scholar]
- 17.Han Z, Li G, Bremner T A, Lange T S, Zhang G, Jemmerson R, Wyche J H, Hendrickson E A. A cytosolic factor is required for mitochondrial cytochrome c efflux during apoptosis. Cell Death Differ. 1998;5:469–479. doi: 10.1038/sj.cdd.4400367. [DOI] [PubMed] [Google Scholar]
- 18.Hengartner M O. Death cycle and swiss army knives. Nature. 1998;391:441–442. doi: 10.1038/35036. [DOI] [PubMed] [Google Scholar]
- 19.Hsu Y-T, Wolter K G, Youle R J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrado A M, Huang Y, Fang G, Liu L, Bhalla K. Overexpression of Bcl-2 or Bcl-XL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996;56:4743–4748. [PubMed] [Google Scholar]
- 21.Itoh N, Nagata S. A novel protein domain required for apoptosis. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 22.Juergensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan Z M, Saxena S, Weichselbaum R, Nalin C, Kufe D W. Role for Bcl-XL as an inhibitor of cytosolic cytochrome c accumulation in DNA-damage induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiefer M C, Brauer M J, Powers V C, Wu J J, Umansky S R, Tomei L D, Barr P J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 25.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins (CAP) form a death-inducing signalling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 27.Kuwana T, Smith J J, Muzio M, Dixit V, Newmeyer D D, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 31.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 32.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. X-ray and NMR structure of human Bcl-XL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 33.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 34.Muzio M, Salvesen G S, Dixit V M. FLICE induced apoptosis in a cell-free system. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 35.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 36.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson W D, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;257:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 39.Oltvai Z N, Korsmeyer S J. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 40.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 41.Orth K, O’Rourke K, Salvesen G S, Dixit V M. Molecular ordering of apoptotic mammalian CED-3/ICE-like proteases. J Biol Chem. 1996;271:20977–20980. doi: 10.1074/jbc.271.35.20977. [DOI] [PubMed] [Google Scholar]
- 42.Pan G, O’Rourke K, Dixit V M. Caspase-9, Bcl-XL and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 43.Peter M E, Kischkel F C, Hellbardt S, Chinnaiyan A M, Krammer P H, Dixit V M. CD95 (APO-1/Fas)-associating signalling proteins. Cell Death Differ. 1996;3:161–170. [PubMed] [Google Scholar]
- 44.Porter A G, Ng P, Janicke R. Death substrates come alive. Bioessays. 1997;19:501–507. doi: 10.1002/bies.950190609. [DOI] [PubMed] [Google Scholar]
- 45.Reed J C. Cytochrome c: can’t live with it—can’t live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 46.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 47.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 49.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasula S M, Ahmand M, Fernandes-Almenri G, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasula S M, Ahmand M, Fernandes-Almenri T, Alnemri E S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 53.Steemans, M., V. Goossens, M. Van de Craen, F. Van Herreweghe, K. Vancompernolle, K. De Vos, P. Vandenabeele, and J. Grooten. A caspase-activated factor (CAF) induces mitochondrial membrane depolarization and cytochrome c release by a nonproteolytic mechanism. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 54.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 55.Tartaglia L A, Ayres T M, Wong G H W, Goeddel D V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 56.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 57.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 58.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 59.Wang K, Yin X-M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Lymphoproliferative disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 61.Wolter K G, Hsu Y-T, Smith C L, Nechushtan A, Xi X-G, Youle R J. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 64.Yang X, Chang H Y, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 65.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist Bad in response to survival factor results in binding to 14-3-3 not Bcl-XL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 66.Zhivotovsky B, Orrenius S, Brustugun O T, Doskeland S O. Injected cytochrome c induces apoptosis. Nature. 1998;391:450–451. doi: 10.1038/35060. [DOI] [PubMed] [Google Scholar]