Abstract
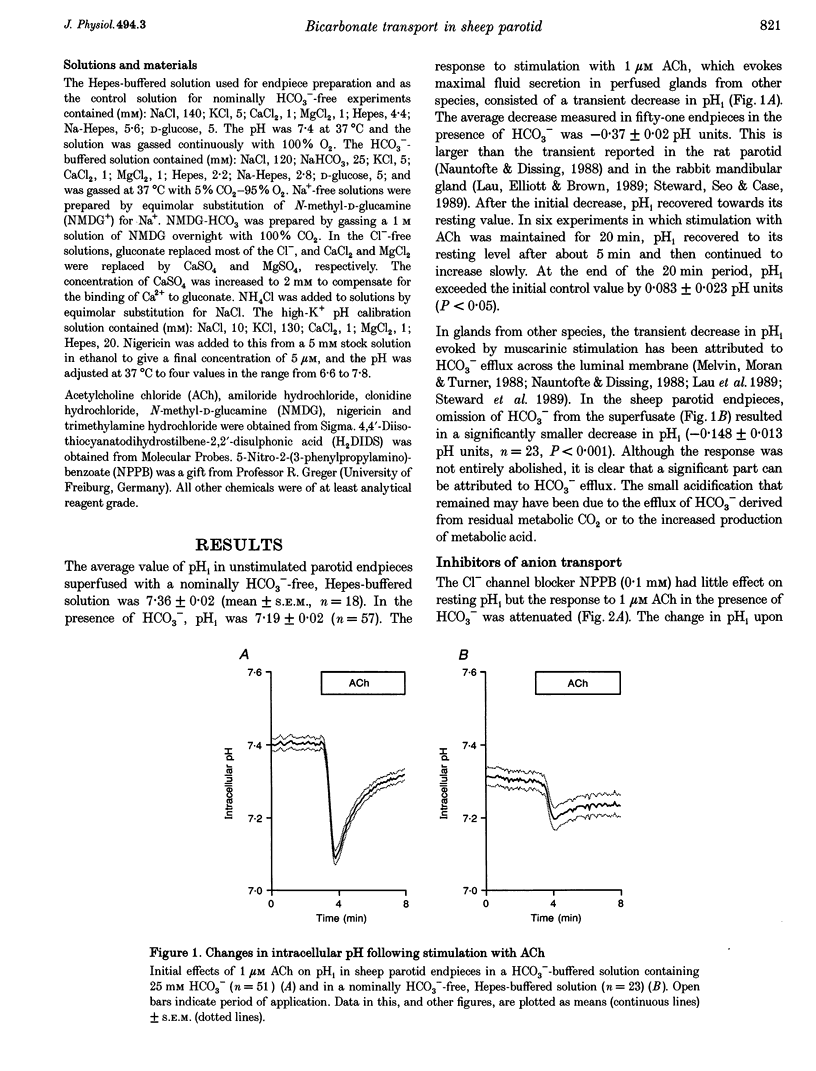
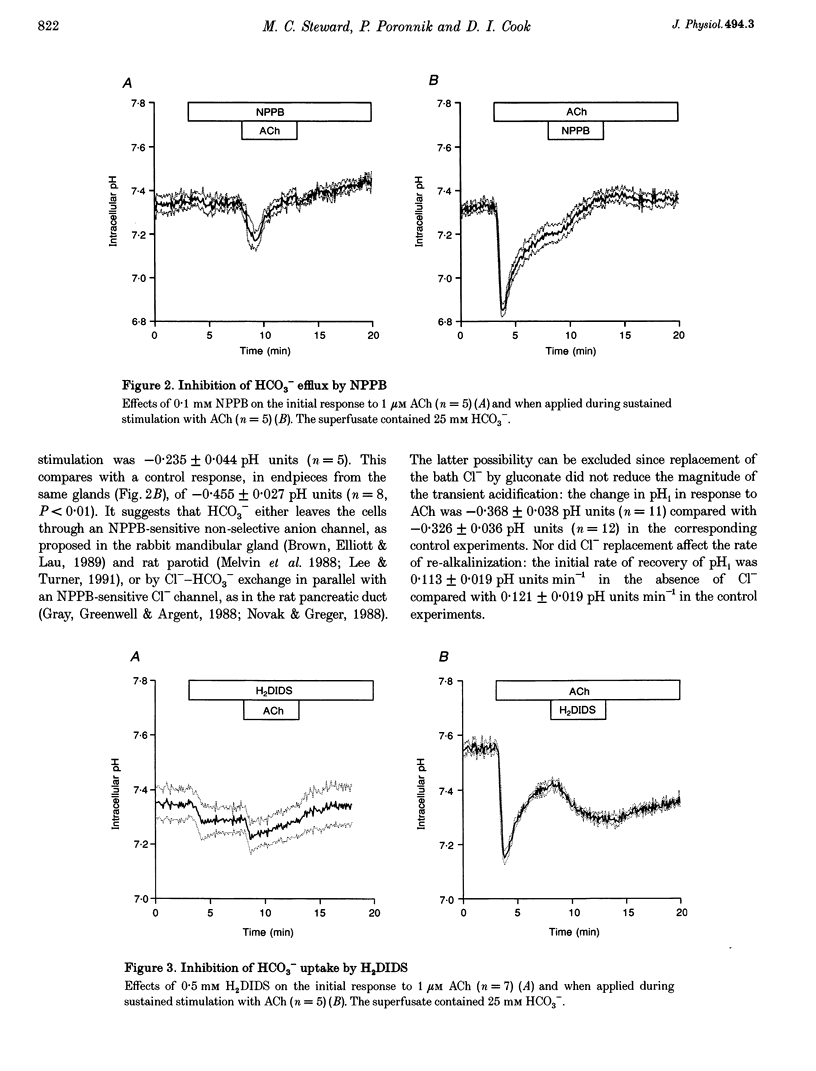
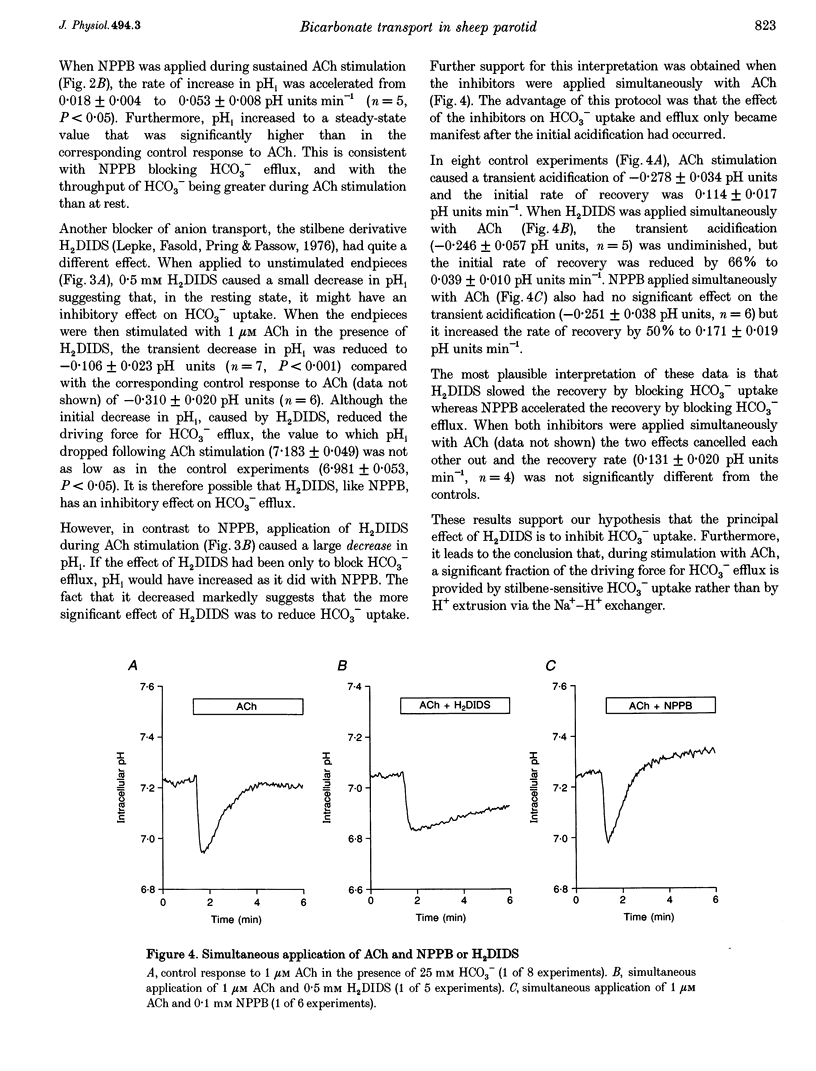
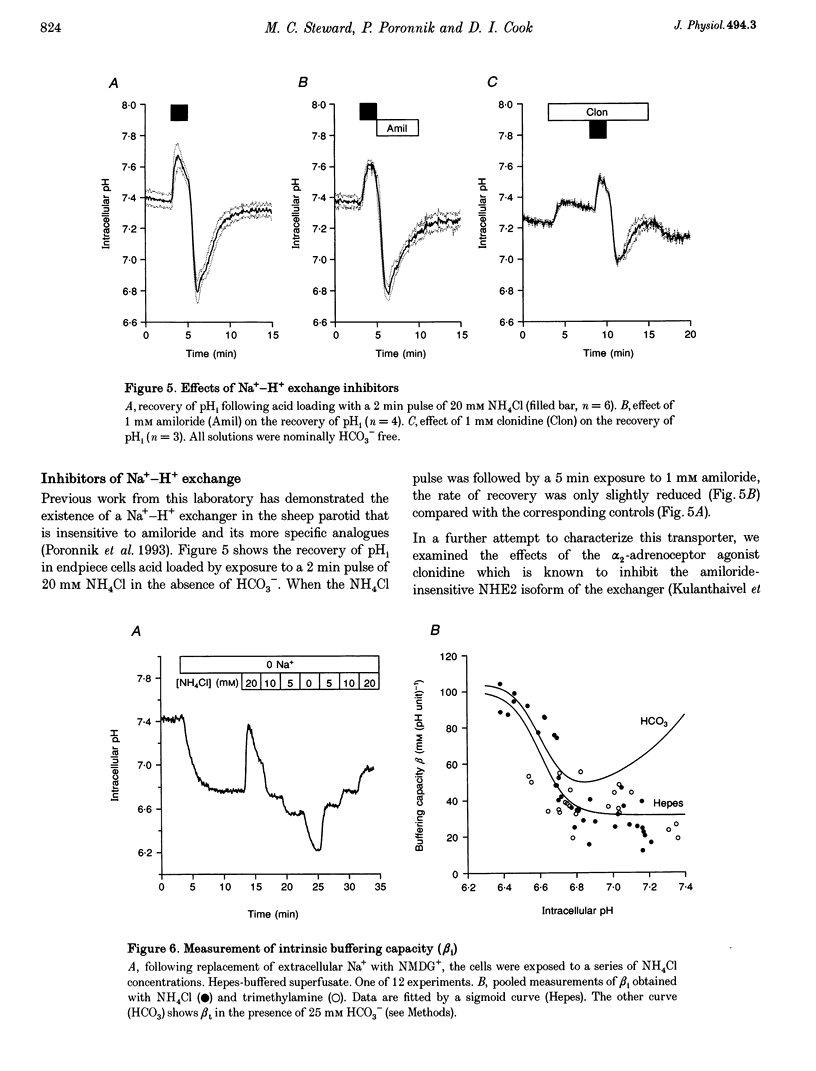
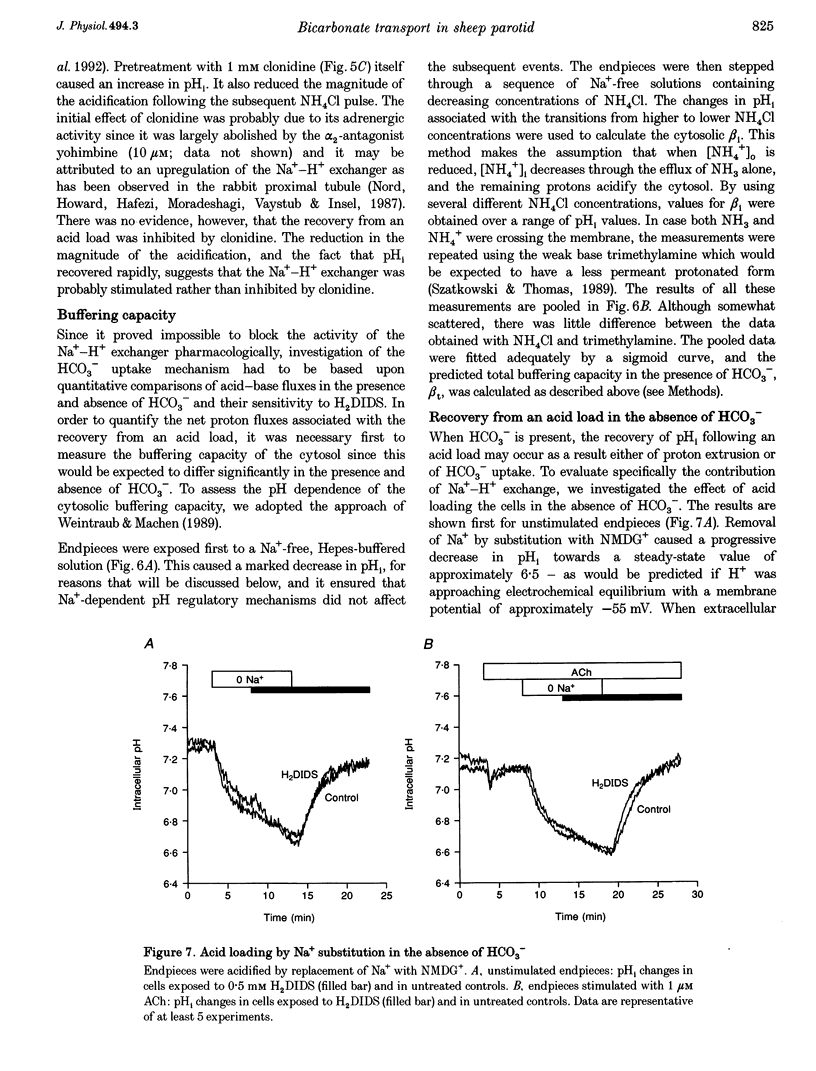
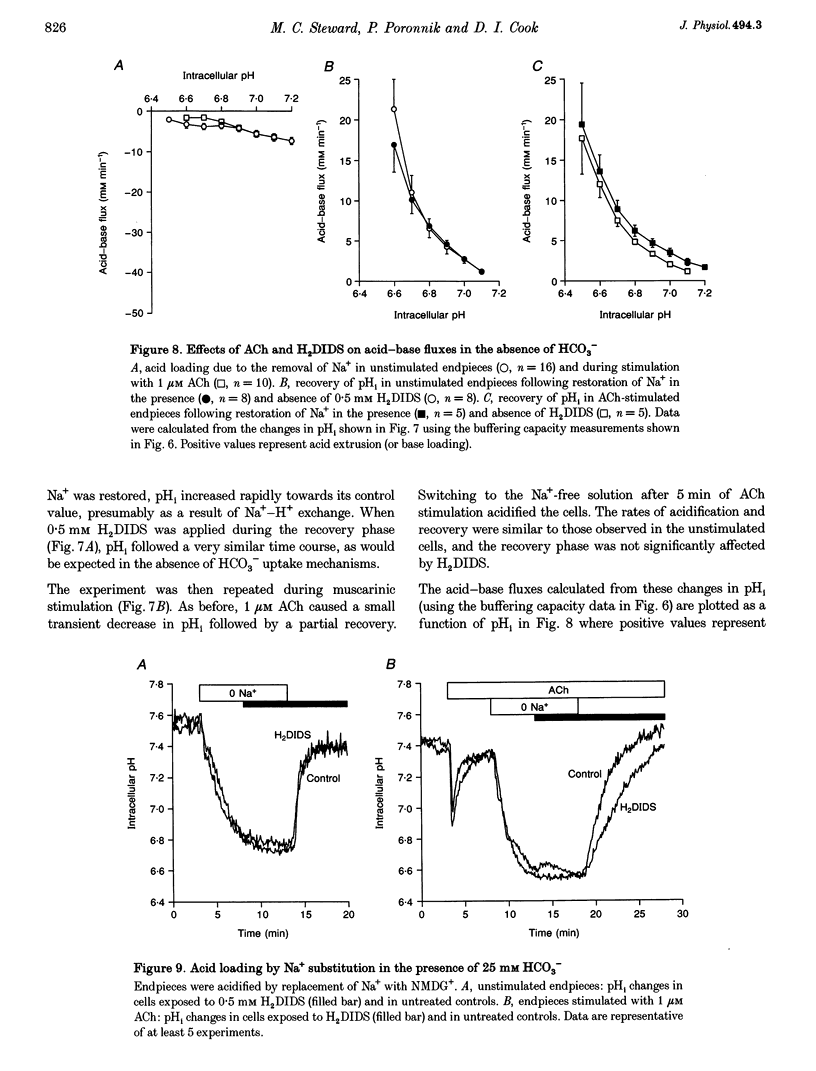
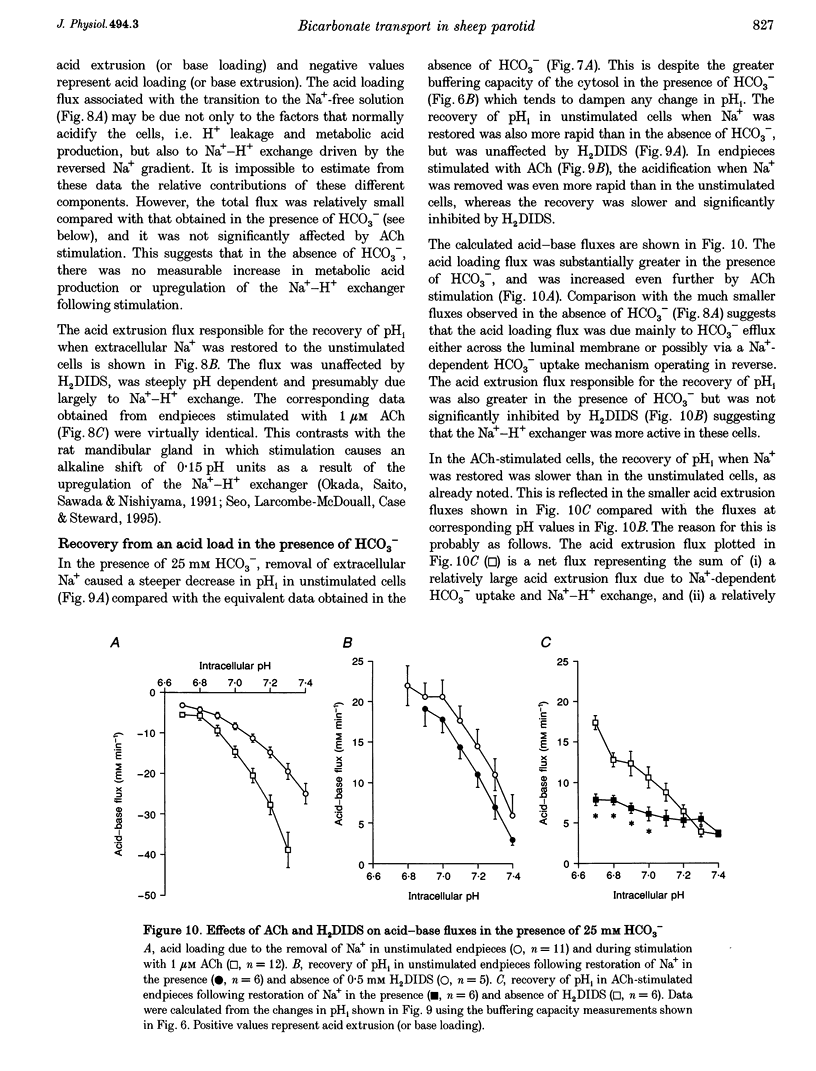
1. Intracellular pH (pH1) was measured by microfluorimetry in secretory endpieces isolated from sheep parotid glands and loaded with the pH-sensitive fluoroprobe 2', 7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF). 2. Stimulation with 1 microM acetylcholine (ACh) caused a large, transient decrease in pH1 of 0.37 +/- 0.02 pH units followed by a slower recovery. The transient, which was reduced by 60% in the absence of HCO3-, could be attributed mainly to HCO3- efflux. During sustained stimulation, pH1 increased to a value that exceeded the resting value by 0.083 +/- 0.023 pH units after 20 min. 3. The anion channel blocker NPPB (0.1 mM) reduced the transient acidification in response to ACh by 48% and raised pH1 during sustained stimulation. Simultaneous application of NPPB and ACh accelerated the re-alkalinization following the initial acidification, indicating that NPPB inhibits HCO3- efflux. 4. The stilbene derivative H2DIDS (0.5 mM) reduced the transient acidification in response to ACh by 76% but caused a marked decrease in pH1 during sustained stimulation. Simultaneous application of H2DIDS and ACh slowed the re-alkalinization following the initial acidification, indicating that the main effect of H2DIDS was to inhibit HCO3- accumulation. 5. In the absence of HCO3-, the recovery from an acid load was unaffected by ACh stimulation. Acid extrusion, although dependent on Na+, was not inhibited by amiloride (1 mM), clonidine (1 mM) or H2DIDS (0.5 mM) and was therefore provisionally attributed to a Na(+)-H+ exchanger isoform other than NHE1 or NHE2. 6. In the presence of HCO3-, the rate of recovery from an acid load was reduced during ACh stimulation, probably as a result of the increased efflux of HCO3-. Acid extrusion was dependent on Na+ and was significantly inhibited by H2DIDS. 7. We conclude that ACh-evoked HCO3- secretion in the sheep parotid gland differs from that in many other salivary glands by being driven predominantly by basolateral Na(+)-HCO3- cotransport rather than by Na(+)-H+ exchange.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal A. M. Electrolyte composition of parotid saliva from sodium-replete red kangaroos (Macropus rufus). J Exp Biol. 1984 Jul;111:225–237. doi: 10.1242/jeb.111.1.225. [DOI] [PubMed] [Google Scholar]
- Beal A. M. Mechanisms of fluid and ion secretion by the parotid gland of the kangaroo, Macropus rufus, assessed by administration of transport-inhibiting drugs. J Comp Physiol B. 1995;165(5):396–405. doi: 10.1007/BF00387310. [DOI] [PubMed] [Google Scholar]
- Beal A. M. The effect of carbonic anhydrase inhibitors on secretion by the parotid and mandibular glands of red kangaroos Macropus rufus. J Comp Physiol B. 1991;161(6):611–619. doi: 10.1007/BF00260752. [DOI] [PubMed] [Google Scholar]
- Blair-West J. R., Fernley R. T., Nelson J. F., Wintour E. M., Wright R. D. The effect of carbonic anhydrase inhibitors on the anionic composition of sheep's parotid saliva. With an appendix on uncatalysed carbon dioxide-water kinetics by P. T. McTigue. J Physiol. 1980 Feb;299:29–44. doi: 10.1113/jphysiol.1980.sp013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Elliott A. C., Lau K. R. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. J Physiol. 1989 Jul;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATS D. A., WRIGHT R. D. Secretion by the parotid gland of the sheep: the relationship between salivary flow and composition. J Physiol. 1957 Mar 11;135(3):611–622. doi: 10.1113/jphysiol.1957.sp005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hunter M., Novak I., Young J. A. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. J Physiol. 1984 Apr;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J. S., Nelson J., Wright R. D., Young J. A. A micropuncture investigation of electrolyte transport in the parotid glands of sodium-replete and sodium-depleted sheep. J Physiol. 1980 Dec;309:429–446. doi: 10.1113/jphysiol.1980.sp013518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci S., Debellis L., Caroppo R., Frömter E. Model of bicarbonate secretion by resting frog stomach fundus mucosa. I. Transepithelial measurements. Pflugers Arch. 1994 Oct;428(5-6):648–654. doi: 10.1007/BF00374589. [DOI] [PubMed] [Google Scholar]
- Fernley R. T., Wright R. D., Coghlan J. P. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett. 1979 Sep 15;105(2):299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Kulanthaivel P., Furesz T. C., Moe A. J., Smith C. H., Mahesh V. B., Leibach F. H., Ganapathy V. Human placental syncytiotrophoblast expresses two pharmacologically distinguishable types of Na(+)-H+ exchangers, NHE-1 in the maternal-facing (brush border) membrane and NHE-2 in the fetal-facing (basal) membrane. Biochem J. 1992 May 15;284(Pt 1):33–38. doi: 10.1042/bj2840033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. R., Elliott A. C., Brown P. D. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C288–C295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Lee S. I., Turner R. J. Mechanism of secretagogue-induced HCO3- and Cl- loss from rat parotid acini. Am J Physiol. 1991 Jul;261(1 Pt 1):G111–G118. doi: 10.1152/ajpgi.1991.261.1.G111. [DOI] [PubMed] [Google Scholar]
- Lee S. I., Turner R. J. Secretagogue-induced 86Rb+ efflux from bovine parotid is HCO3- dependent. Am J Physiol. 1993 Jan;264(1 Pt 2):R162–R168. doi: 10.1152/ajpregu.1993.264.1.R162. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Manganel M., Turner R. J. Agonist-induced activation of Na+/H+ exchange in rat parotid acinar cells. J Membr Biol. 1989 Oct;111(2):191–198. doi: 10.1007/BF01871782. [DOI] [PubMed] [Google Scholar]
- Melvin J. E., Moran A., Turner R. J. The role of HCO3- and Na+/H+ exchange in the response of rat parotid acinar cells to muscarinic stimulation. J Biol Chem. 1988 Dec 25;263(36):19564–19569. [PubMed] [Google Scholar]
- Nauntofte B., Dissing S. Cholinergic-induced electrolyte transport in rat parotid acini. Comp Biochem Physiol A Comp Physiol. 1988;90(4):739–746. doi: 10.1016/0300-9629(88)90693-7. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Howard M. J., Hafezi A., Moradeshagi P., Vaystub S., Insel P. A. Alpha 2 adrenergic agonists stimulate Na+-H+ antiport activity in the rabbit renal proximal tubule. J Clin Invest. 1987 Dec;80(6):1755–1762. doi: 10.1172/JCI113268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Novak I., Young J. A. Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch. 1986 Dec;407(6):649–656. doi: 10.1007/BF00582647. [DOI] [PubMed] [Google Scholar]
- Okada M., Saito Y., Sawada E., Nishiyama A. Microfluorimetric imaging study of the mechanism of activation of the Na+/H+ antiport by muscarinic agonist in rat mandibular acinar cells. Pflugers Arch. 1991 Oct;419(3-4):338–348. doi: 10.1007/BF00371116. [DOI] [PubMed] [Google Scholar]
- Poronnik P., Schumann S. Y., Cook D. I. HCO3(-)-dependent ACh-activated Na+ influx in sheep parotid secretory endpieces. Pflugers Arch. 1995 Apr;429(6):852–858. doi: 10.1007/BF00374810. [DOI] [PubMed] [Google Scholar]
- Poronnik P., Young J. A., Cook D. I. Na(+)-H+ exchange in sheep parotid endpieces. Apparent insensitivity to amiloride. FEBS Lett. 1993 Jan 11;315(3):307–312. doi: 10.1016/0014-5793(93)81184-2. [DOI] [PubMed] [Google Scholar]
- Seo J. T., Larcombe-McDouall J. B., Case R. M., Steward M. C. Modulation of Na(+)-H+ exchange by altered cell volume in perfused rat mandibular salivary gland. J Physiol. 1995 Aug 15;487(1):185–195. doi: 10.1113/jphysiol.1995.sp020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. C., Seo Y., Case R. M. Intracellular pH during secretion in the perfused rabbit mandibular salivary gland measured by 31P NMR spectroscopy. Pflugers Arch. 1989 Jun;414(2):200–207. doi: 10.1007/BF00580964. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989 Feb;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Weintraub W. H., Machen T. E. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989 Sep;257(3 Pt 1):G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- Wright R. D., Blair-West J. R., Nelson J. F. Effects of ouabain, amiloride, monensin, and other agents on ovine parotid secretion. Am J Physiol. 1986 Mar;250(3 Pt 2):F503–F510. doi: 10.1152/ajprenal.1986.250.3.F503. [DOI] [PubMed] [Google Scholar]


