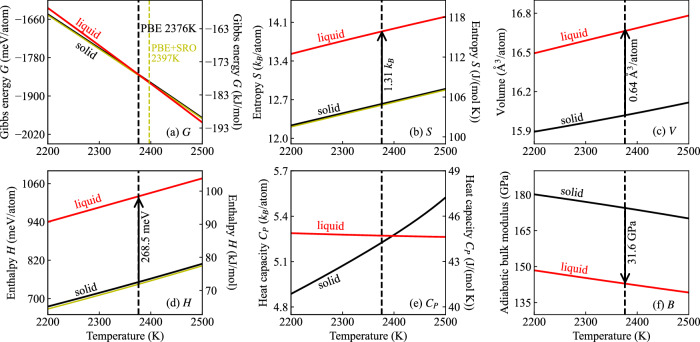
Fig. 4. Temperature dependence of thermodynamic properties of solid and liquid TaVCrW from GGA-PBE calculations.
a Gibbs energy (G), (b) entropy (S), (c) volume (V), (d) enthalpy (H), (e) heat capacity (CP), and (f) bulk modulus (B). The yellow lines in (a), (b), and (d) represent the solid Gibbs energy, entropy, and enthalpy, including the SRO contribution, which slightly stabilizes the solid phase and results in a higher melting point of 2397 K.