Abstract
Stride velocity 95th centile (SV95C) is a wearable-derived endpoint representing the 5% fastest strides taken during everyday living. In July 2023, SV95C received European Medicines Agency (EMA) qualification for use as a primary endpoint in trials of patients with Duchenne muscular dystrophy (DMD) aged ≥ 4 years—becoming the first digital endpoint to receive such qualification. We present the data supporting this qualification, providing insights into the evidentiary basis of qualification as a digital clinical outcome assessment. Clinical trials, natural history studies, and patient surveys (ages 5 − 14 years) showed that SV95C is accurate, valid, reliable, sensitive, and clinically meaningful. SV95C significantly correlated with traditional DMD assessments, increased rapidly after steroid initiation (0.090 m/s 3 months post-treatment), and declined steadily in patients on stable steroid regimens. Compared with traditional assessments, SV95C demonstrated earlier sensitivity to disease progression (3 vs 9 months) and greater sensitivity at 12 months. Distribution- and anchor-based approaches revealed a change of − 0.10 to − 0.20 m/s as clinically meaningful. The EMA qualification of SV95C illustrates the willingness of regulators to accept novel digital endpoints for drug approval, setting an important precedent for the evidentiary basis of regulatory digital endpoint qualification that could transform clinical development in disorders affecting movement.
Keywords: Stride Velocity 95th Centile, Digital endpoints, Wearables, Duchenne muscular dystrophy, Regulatory qualification, V3 framework
Subject terms: Neurological disorders, Neuromuscular disease, Clinical trial design, Bioinformatics, Biomedical engineering, Musculoskeletal system
Introduction
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disease for which there is no cure1, and DMD clinical trials face major challenges2,3. The number of patients available for clinical trials in rare diseases is limited, with patients often geographically dispersed, leading to recruitment challenges and complex study designs3,4. Moreover, the gold-standard functional endpoints in DMD (6-min Walk Distance [6MWD], North Star Ambulatory Assessment [NSAA], and 4-stair Climb [4SC]) are performed episodically in a controlled clinical environment, limiting their ability to capture sporadic real-world function, measure endurance, and quantify linear changes5. Hence, large participant numbers and long study periods are often required to sufficiently power trials5.
Digital health technology (DHT) may improve trial design by providing non-invasive, objective, continuous assessment of aspects of patient health in daily life6. Digital endpoints collected by validated and suitable technology during activities of daily living reduce the burden of traveling to clinical sites and completing assessments over extended periods6. To realize these benefits, it is vital that fit-for-purpose DHTs undergo extensive validation within the appropriate context of use7. Until recently, no digital endpoint was qualified by regulatory agencies for use as a primary outcome in clinical trials.
Stride velocity 95th centile (SV95C) is a variable, derived from a wearable DHT, that represents the top ambulation speed of a patient—measured as the velocity of the 5% most rapid strides taken in a real-world setting8,9. In 2019, SV95C (measured by a suitable and valid device) received regulatory European Medicines Agency (EMA) qualification as a secondary endpoint in clinical trials of patients with DMD aged ≥ 5 years10,11. Subsequently, a substantially larger data set was submitted to the EMA. Based on this data set, in July 2023, the EMA qualified SV95C as a primary endpoint for use in clinical trials of patients with DMD aged ≥ 4 years—making it the first digitally derived outcome measure to receive formal regulatory qualification as a primary endpoint in clinical trials of any indication12. SV95C has been included as an endpoint in multiple clinical trials of DMD, including SPITFIRE (NCT03039686), DYSTANCE 51 (NCT03907072), EMBARK (NCT05096221), INSPIRE DUCHENNE (NCT06138639), FORWARD-53 (NCT04906460), and DELIVER (NCT05524883). We present the evidentiary data that supported the EMA approval of SV95C as a primary endpoint.
Discussion and observations
Analytical validation
In healthy participants (N = 8), the wearable detected 98.7% of strides in a motion capture room. At the fast-walking speed, the mean stride speed was 152.88 cm/s, with a mean difference of 0.01 cm/s and a root-mean-square difference of 1.02 cm/s versus the mean stride speed measured by the motion capture system.
In patients with DMD (N = 23), the difference in 6-min Walk Distance (6MWD) measured by physiotherapists versus the wearable was 0.75 ± 8.9 m (0.24%; mean total distance: 307.6 ± 103.5 m).
Compliance
Device-wearing compliance was generally good. At baseline, 88% of patients with DMD (111/125) and 85% of healthy individuals (56/66) achieved or exceeded the optimum recording duration of 180 h, whereas the minimum recording threshold of 50 h was reached or exceeded by 100% of patients with DMD (125) and 97% (64/66) of healthy individuals.
Clinical validation
Convergent validity
SV95C was significantly correlated with 6MWD, NSAA, and 4SC at baseline and months 3, 6, 9, and 12 (Supplementary Fig. S1, Supplementary Table S1), suggesting that SV95C is closely related to established clinical outcome assessments in DMD.
Test–retest reliability
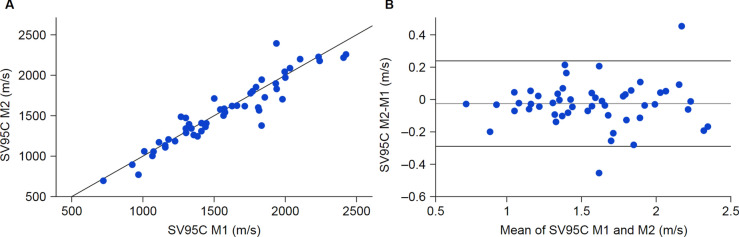
Intraclass correlation coefficient (ICC) analyses of SV95C in patients with DMD aged 5 − 14 years supported excellent reliability, including in younger patients, with results comparable to or better than those previously reported for 6MWD (ICC 0.75 − 0.92) and NSAA (ICC 0.86 − 0.98)13–16. The ICC based on two successive SV95C recordings collected 1 month apart from patients was 0.970 (95% confidence interval 0.947 − 0.983) (Fig. 1). Similar results were obtained when stratifying by age group (ICC 0.956 for ages 5 − 7 years; 0.957 for ages 8 − 14 years).
Fig. 1.
Comparison of SV95C values measured 1 month apart in two successive recording periods. (A) SV95C measured during month 1 versus month 2, (B) Corresponding Bland–Altman plot. The gray line represents the mean of the difference between SV95C measured at month 2 and month 1 (mean SV95C difference –0.024 m/s). Dark lines represent the mean difference between the measurements ± 1.96 SD (SD 0.136 m/s). M month, SD standard deviation, SV95C stride velocity 95th centile.
Source: Qualification Opinion for Stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne Muscular Dystrophy studies © European Medicines Agency, 2022.
SV95C demonstrated good stability and low variability. An intra-patient variability analysis of 28 patients who wore the device for ≥ 1800 h (Fig. 2A) showed an average variability of 6.38% for 50-h periods and 4.41% for 180-h periods. Below 50 h of recording, intra-patient variability increased dramatically5, which informed the criterion that the device should be worn for ≥ 50 h during a predefined recording period. Further, a cutoff of 50 h may account for missing data from patients who are poorly compliant during trials and permit more reliable longitudinal analyses. Differences in SV95C between patients and controls were observed at all ages studied (Fig. 2B). Figure 2C shows the influence of time of recording.
Fig. 2.
SV95C variability, SV95C as a function of age, and influence of time of recording on SV95C in patients with DMD and controls (A) The variability of SV95C as a function of the number of recorded hours used to compute the variable. The variability was computed as follows: for each recording duration of X hours, the patient’s data were regrouped into periods of X hours and the variance of SV95C computed on each period was estimated. The bold line represents the mean across patients for each recording duration. The gray region is the mean ± 1 SD, (B) Individual SV95C values in patients and controls of different ages with > 50 recorded hours, (C) Comparisons of daytime versus evening SV95C recordings and weekday versus weekend SV95C recordings. Wilcoxon test between AM/PM distributions and weekday/weekend distributions in patients with DMD and controls. *p < 0.01. P values were as follows: 0.117 for AM/PM, DMD; 0.616 for AM/PM, controls; 0.00631 for week/weekend, DMD; 2.81e-07 for week/weekend, controls. DMD Duchenne muscular dystrophy, SD standard deviation, SV95C stride velocity 95th centile.
Known-groups validity
SV95C also differed significantly between patients with DMD and controls, with findings similar to those previously observed for 6MWD and NSAA17,18. Patients with DMD had lower median SV95C values (1.563 m/s; N = 125) than controls (2.713 m/s; N = 66; p < 0.001). Similarly, the median 6MWD was significantly lower in patients (389.0 m vs. 605.0 m; p < 0.001), and the median 4SC was significantly higher in patients (3.40 s vs. 1.27 s; p < 0.001).
Responsiveness
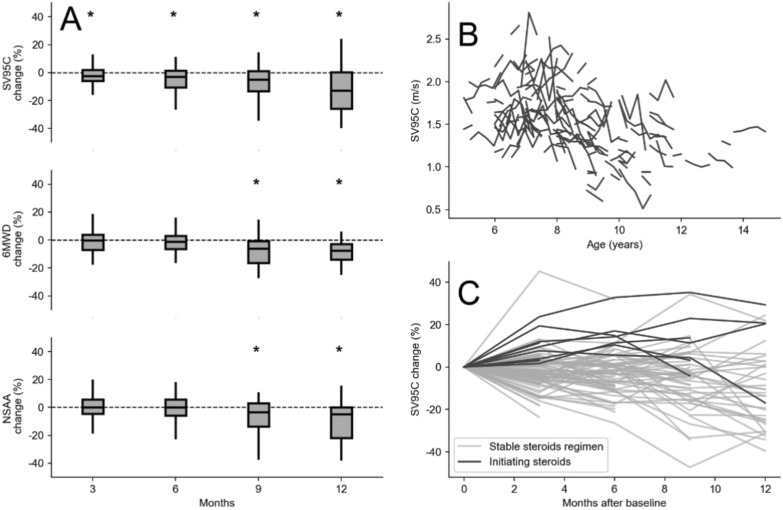
The change in SV95C over time suggests a progressive loss of maximal speed that is documentable over as little as 3 months, compared with 9 months for the other measures in the population considered. In patients with DMD on a stable corticosteroid regimen, the median relative change from baseline (%) in SV95C was − 2.500 at 3 months (p = 0.0019; N = 91), − 3.094 at 6 months (p = 0.0009; N = 64), − 5.155 at 9 months (p = 0.0005; N = 58), and − 12.842 at 12 months (p = 0.0003; N = 34) (Fig. 3). In the same population, significant differences from baseline in 6MWD and NSAA were identified after 9 months (Fig. 3). For the 6MWD, the median relative changes were − 0.235 at 3 months (p = 0.5443; N = 47), − 1.102 at 6 months (p = 0.2032; N = 56), − 6.144 at 9 months (p = 0.0016; N = 30), and − 7.661 at 12 months (p = 0.0007; N = 24). For the NSAA, these values were 0.000 at 3 months (p = 0.9304; N = 51), 0.000 at 6 months (p = 0.8737; N = 42), − 3.391 at 9 months (p = 0.0153; N = 32), and − 4.963 at 12 months (p = 0.0192; N = 18). Median relative changes from baseline (%) in 4SC (not pictured in Fig. 3 due to high variability) were 6.393 at 3 months (p = 0.0120; N = 51), 10.959 at 6 months (p = 0.0019; N = 41), 13.719 at 9 months (p = 0.0066; N = 32), and 33.548 at 12 months (p = 0.0182; N = 18). Respective standardized response means (SRMs) for changes at months 3, 6, 9, and 12 were − 0.22, − 0.38, − 0.46, and − 0.74 for SV95C; − 0.05, − 0.19, − 0.69, and − 0.72 for 6MWD; 0.00, − 0.16, − 0.46, and − 0.66 for NSAA; and 0.27, 0.48, 0.54, and 0.60 for 4SC.
Fig. 3.
Responsiveness to change and comparison with other measures. (A) Median change from baseline in SV95C, 6MWD, and NSAA in patients with DMD who were on a stable steroid regimen. Asterisks denote significant changes from baseline. Respective participant numbers at months 3, 6, 9, and 12 were n = 91, n = 64, n = 58, and n = 34 for SV95C; n = 47, n = 56, n = 30, and n = 24 for 6MWD; and n = 51, n = 42, n = 32, and n = 18 for NSAA, (B) Relative evolution of SV95C according to age at baseline. Individual patient trajectories show SV95C plotted as a function of age, (C) Baseline-normalized SV95C trajectories showing relative evolution in patients initiating corticosteroids (darker line) versus those on a stable steroid regimen. Relative evolution is represented as the percentage of the baseline value. 6MWD 6-min Walk Distance, DMD Duchenne muscular dystrophy, NSAA North Star Ambulatory Assessment, SV95C stride velocity 95th centile.
These results indicate that SV95C may be more sensitive to disease progression over 12 months than the 6MWD and NSAA, enabling earlier detection with fewer patients. These findings have promising implications for future study designs, as SV95C could enable shorter trials and reduce the placebo burden.
In a sample of 11 patients with DMD who initiated corticosteroids at study start, a significant positive change in SV95C, indicating treatment benefit, was observed as early as 3 months post-treatment (0.090 m/s; p = 0.003; SRM 1.05) and at 6 months (0.211 m/s; p = 0.018; SRM 1.44). No additional positive changes were observed at 9 months.
Meaningful change
Results of the meaningful change analyses suggest that an SV95C change of at least ≈–0.10 m/s in patients with DMD would be beyond measurement error evaluated at 0.07 m/s, while a − 0.10 to − 0.20 m/s change would be meaningful5. These changes were observed within 9 months in steroid-treated patients (negative) and within 6 months after steroid initiation (positive).
For anchor-based estimates, an overall strong cross-sectional correlation between SV95C and Clinical Global Impression of Change (CGI-C) was observed at week 48 (Spearman correlation − 0.816; p = 0.001), with a trend toward lower SV95C with worsening CGI-C. While there was no correlation between change in SV95C and CGI-C at week 48, most patients with unchanging or worsening global clinical states (clinician-reported) showed a negative change in SV95C that exceeded the minimal detectable change (MDC) at the 80% confidence level (CL) of 0.127 m/s. Similarly, there was a significant cross-sectional correlation between SV95C and Pediatrics Outcomes Data Collection Instrument (PODCI) “transfers and basic mobility” at week 48 (Spearman correlation 0.611; p = 0.015), but none between the changes from baseline. Nevertheless, all but one child with a lower parent-reported “transfers and basic mobility” domain at week 48 versus baseline exhibited a decrease in SV95C.
Overall, patients whose CGI-C responses indicated improvement (“minimally improved,” “much improved,” or “very much improved”) had the smallest decrease in median SV95C change over 48 weeks (− 0.025 m/s; Supplementary Table S2). No patients were categorized as improved based on the PODCI (≥ 10% gain). The largest SV95C reductions were in patients categorized as worsened based on the CGI-C (− 0.280 m/s) and PODCI (− 0.245 m/s). Considering PODCI and CGI-C, respectively, the median SV95C change considered stable or worsened was − 0.145 m/s and − 0.070 m/s.
The anchor analyses based on traditional endpoints also supported a meaningful change threshold (MCT) of ~ 0.1 m/s; patients with worsening NSAA (median change of − 2 to − 3 points) after 48 weeks had a median SV95C change of − 0.115 m/s (N = 4), while those who worsened on 6MWD (change of − 30 to − 40 m) had a median SV95C change of − 0.130 m/s (N = 3).
For distribution-based thresholds, the standard error of measurement of SV95C was 0.070 m/s in patients aged 5 − 14 years and 0.156 m/s in controls aged 6 − 14 years (Supplementary Table S3). The MDC of SV95C at the 80% CL was 0.127 m/s in patients and 0.282 m/s in controls. The half-standard deviation estimate in patients was 0.191 m/s.
Qualitative results
Stride velocity selection
Discussions with clinician experts highlighted the relevance of step-related stride length and stride speed variables, based on their independence from social and environmental parameters, and the importance of maximum ability.
Healthcare professional perspectives
During the public consultation, most clinicians agreed that common primary endpoints in DMD clinical trials are highly dependent on patient motivation, fatigue, concentration, well-being, growth, and intellectual maturation.
All healthcare professionals who completed the online wearable survey (N = 38) and most solicited experts acknowledged the value of measuring maximal ambulation speed to evaluate changes in walking abilities. Most healthcare professionals acknowledged the utility of a wearable for detecting these changes.
Patient and caregiver perspectives
Comments received from patient organizations are in Supplementary Results A − D.
Results from the global online survey indicated that limiting falls, self-transfer ability, and walking ability were considered “very important” to patients and caregivers (Fig. 4).
Fig. 4.
Importance of ambulation aspects according to patients with DMD and caregivers of patients with DMD, stratified by walking ability. Percentages are based on n = 44 ambulant and n = 38 non-ambulant participants with DMD who answered these questions.
Source: Qualification Opinion for Stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne Muscular Dystrophy studies © European Medicines Agency, 2022. DMD Duchenne muscular dystrophy.
Patients cited walking as the function they would most like restored in a clinical trial. Most (57.78%; 26/45) stated that a change in top walking speed represents improved ambulation.
To assess mobility during a clinical trial, most respondents preferred a wearable device in a real-world setting over a regular assessment in a clinic-based setting. Over 70% (55/78) noted that a wearable would make trial participation more attractive. Over 75% (61/77) were willing to use the device, and 44.6% (33/74) were willing to wear it continuously throughout a trial.
EMA qualification: implications, benefits, and challenges
These data supported the first regulatory agency qualification of a digital outcome measure as a primary endpoint for any indication12. This landmark decision was based on a well-defined context of use, analytical and clinical validation (consistent with the Digital Medicine Society 3-component [‘V3’] framework), and an extended data set derived from clinical trials conducted by different sponsors.
The EMA qualifies an outcome and not a device, but precisely defines how the outcome must be measured11. Therefore, EMA qualification of SV95C for use as a primary endpoint sets an important regulatory precedent, indicating that health authorities are willing to accept data acquired with novel DHTs and digital endpoints for drug approval purposes. The US Food and Drug Administration Drug Development Tool qualification procedure is ongoing.
While the validation undertaken for SV95C in the context of DMD renders the DHT largely disease-agnostic, investigations are ongoing to develop and validate disease-specific variables in other diseases and conditions, including facioscapulohumeral muscular dystrophy, spinal muscular atrophy, multiple sclerosis (NCT04882891), and Angelman syndrome (NCT05100810), as well as non-ambulant patients with DMD19–22.
The use of wearables in clinical trials can provide many benefits. Wearable devices can measure additional real-world variables (e.g., stair climbing, walking perimeter) to create a platform of outcomes and offer an opportunity to further bolster clinical trial conclusions. By capturing real-world function outside of brief hospital visits, wearables can reduce the need for frequent travel to in-clinic assessments, expanding the available population for clinical trials.
Wearable technologies also present specific challenges related to technical reliability, data privacy, and patient compliance. The risk of technical failure should be managed through proper design, testing, and quality control prior to equipping patients. Patient, caregiver, and investigator education and support are also paramount to the success of DHT deployment, especially to obtain robust compliance. Regional differences in data protection legislation and DHT classification can be another hurdle. It may also be difficult to demonstrate the content validity of high-sensitivity digital endpoints to patients, and patients may be unaware of the specific value provided by DHTs.
These studies were not designed to assess compliance in the general population. The outstanding compliance results were positively affected by well-trained specialists and exclusion of patients who refused to wear the device prior to joining the study. The reduction in population size at later timepoints was not due to a lack of patient compliance with device use but rather to the interruption or early termination of some of the studies comprising the data set.
Notably, SV95C was initially clinically defined, then validated; it is likely that, in the future, machine learning will play a role in defining digital outcomes23. SV95C is also a single digital endpoint, and may not represent an entire functional domain in the way that NSAA has been designed to. The use of multiple digital endpoints to evaluate gait health could provide additional value in this context; for children with DMD who are progressing toward the loss of ambulation, the association of different digital endpoints (e.g., characterizing upper and lower limb function) may aid in follow-up even after ambulation is lost. Additionally, the evidentiary data submitted to the EMA only included individuals aged ≥ 4 years. Finally, while the wearable described herein is fit-for-purpose, a different wearable with equal performance and usability may be preferred by any given patient.
Conclusions
The evidence presented here shows that SV95C is accurate, reliable, sensitive to change, and clinically meaningful for use as a primary endpoint in clinical trials of ambulant patients with DMD. This endpoint has the potential to transform clinical development in DMD and potentially other neuromuscular diseases and neurodevelopmental conditions.
Methods
Data sources
Natural history and in-clinic data from nine clinical trials (N = 148 ambulant patients with DMD and N = 74 age-matched controls) are presented in Table 124.
Table 1.
Study participants.
| Study name | Number of participantsa | Inclusion criteria | SV95C details | |
|---|---|---|---|---|
| CE-A | Controls | 8 | No muscle condition | Ankle/wrist-worn sensor. Stride-level data compared with motion capture during fast, slow, and normal walking |
| CE-B | Patientsb | 23 | Stable corticosteroids | Ankle/wrist-worn sensor. Stride-level data compared with physician-reported 6MWD |
| NHS-A24 | Patients | 2 | Starting corticosteroids |
Ankle/wrist-worn sensor Continuous recording (up to 13 months)c |
| Controls24 | 62 | No muscle condition |
Ankle/wrist-worn sensor 30-day recording periods 2 recording periods, 1 year apart |
|
| NHS-B | Patients | 13 | Stable (n = 11) or starting (n = 2) corticosteroids |
Ankle/ankle-worn sensor Continuous recordingc |
| Controls | 4 | No muscle condition |
Ankle/ankle-worn sensor 30-day recording periods 2 recording periods, 1 year apart |
|
| NHS-C | Patients | 11 | Stable corticosteroids |
Ankle/wrist-worn sensor Continuous recording (up to 9 months)c |
| CT-A25 | Patients | 34 |
DMD gene deletion amenable to exon 53 skipping Stable corticosteroids |
Ankle/wrist-worn sensor Continuous recording (up to 20 months)c |
| CT-B26 | Patients | 51 | Stable corticosteroids |
Ankle/ankle-worn sensor 45-day recording periods Up to 5 recording periods, every 3 months |
| CT-C | Patients | 7 |
DMD gene deletion amenable to exon 51 skipping Stable corticosteroids |
Ankle/wrist-worn sensor 30-day recording periods 1 baseline recording period |
| In-clinic | Patients | 7 | Starting corticosteroids |
Ankle/wrist-worn sensor Continuous recording (up to 24 months)c |
Data included are from both drug and placebo arms.
6MWD 6-min Walk Distance, CE controlled environment, CT clinical trial, DMD Duchenne muscular dystrophy, NHS natural history study, SV95C stride velocity 95th centile. Source: Qualification Opinion for Stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne Muscular Dystrophy studies © European Medicines Agency, 2022.
aBaseline numbers; subsets were used for longitudinal analyses.
bAdditional patient cohort used for analytical validity (not included in N = 125).
cData from 30-day contiguous recording periods are analyzed.
All studies herein were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The experimental protocols were approved by the respective institutional committee for each of the studies outlined in the EMA qualification opinion. Ethics committee approval was obtained at each participating site (Liège Ethics Committee for NHS-A and NHS-B and Paris VI Ethics Review Board for NHS-C, NatHis-DMD and In-clinic); CT-C was approved by London—Chelsea Research Ethics Committees at each UK site, institutional review boards at each US site, and ethics boards at the different sites in Belgium (Commissie voor Medische Ethiek, Comité d'éthique), Canada (HSREB), Czechia, France (CPP), Italy (Comitato Etico), and Sweden (Etikprövningsmyndigheten). Prior to performing any procedures required for these studies, participants provided informed consent/assent and written informed consent was obtained from a parent or legal guardian. No identifying information or images are included in this manuscript.
SV95C
SV95C represents the minimum velocity of the 5% most rapid strides taken in a real-world setting (i.e., the point at which 95% of strides are slower and 5% of strides are faster). SV95C is derived from data collected passively by sensors on an ankle-worn wearable device matching the EMA technical requirements12. All data submitted to the EMA for qualification of SV95C as a primary endpoint were acquired using the Class I ActiMyo system, which is unique in its ability to measure individual strides. The wearable consists of three parts: a data acquisition system, a software platform for data storage, and analysis software. A smaller, new-generation model, Syde, is now also available.
The wearable is a CE-labeled medical device, indicating conformity with European standards of health, safety, and environmental protection (See additional details in Supplementary Methods A). The wearable uses two watch-like sensors and a docking station. Each sensor contains a tri-axial accelerometer, a gyrometer, ≥ 1 magnetometers, and a barometer to respectively record the linear acceleration, angular velocity, magnetic field of movement (in all directions), and barometric altitude. A dedicated algorithm detects strides of any pace.
The beginning and end of a stride is identified using a model that links ankle acceleration and angular velocity based on biomechanic principles. The ankle trajectory is reconstructed during the stride, allowing for very strong specificity of stride detection in life-like conditions and quantification of other stride parameters, like length, speed, and height.
The patient puts on the device in the morning, wearing one device near the ankle and the other on the second ankle or the wrist (a wrist device is not used for the estimation of SV95C; only one ankle-worn device is needed to compute SV95C). Data are collected passively and stored in an internal memory; these data are then transferred to a docking station every night when the patient removes and charges the device.
From the docking station, data are transferred securely, without being linked to patient ID information, to a dedicated secure web cloud or stored on an internal USB drive for up to 3 months.
Validation of SV95C
The Digital Medicine Society V3 framework for evaluating whether digital clinical measures are fit for purpose includes verification, analytical validation, and clinical validation27. SV95C was validated for use as a primary endpoint in line with this framework, although validation occurred before V3 was published. The EMA focused on clinical validation for this procedure; analytical validation was accepted during the secondary endpoint qualification. Verification was performed on the algorithms that estimate SV95C, and consisted of designing and conducting automated or human-operated tests of the key function required by the algorithms.
Analytical validation
Analytical validation assesses the precision and accuracy of a DHT to derive the clinical features (in this case, stride velocity) in the context of use.
Healthy population
The objective of the analytical validation of SV95C was to confirm that the intermediate elements leading to the computation of SV95C were measured accurately. To that end, velocities of individual strides were acquired simultaneously by motion capture equipment and the wearable device, then compared to assess the level of precision of the wearable device. The Motion Capture System was used as a reference based on its high precision in evaluating stride speed. To determine the precision of stride detection and the accuracy of stride reconstruction in different situations, stride velocity in healthy participants (N = 8) was measured by the wearable as participants walked on three defined trajectories (clockwise turns, anticlockwise turns, and figure eights) at three gait cadences (slow, normal, and fast) in a motion capture room equipped with an OptiTrack Motion Capture System and 12 Primex 13W cameras28. Stride number and stride speed measured by the wearable were compared with stride number and speed measured by the motion capture room.
Patient population
In patients with DMD (N = 23; 31 tests) with varying clinical conditions, the 6MWD (an established clinical outcome assessment in DMD) measured by physiotherapists was compared with that calculated by the wearable.
Clinical validation
Convergent validity
Convergent validity reflects how much a score on one measure is associated with scores on other measures capturing the same construct. The convergent validity of SV95C was assessed in 107 patients with DMD by cross-correlating (parametric [Pearson’s] and non-parametric [Spearman’s] correlations]) SV95C with other clinical outcome assessment measures—6MWD, NSAA, and 4SC—from the same patients at 3, 6, 9, and 12 months.
Briefly, the 4SC is a measure of how long (in seconds) it takes a person to climb 4 stairs29, the 6MWD is the distance in meters a person walks in 6 min30, and the NSAA is a 17-item assessment of motor function, where each item is scored from 0–2 and the total score ranges from 0–34 (higher scores represent better function)18.
Test–retest reliability
Test–retest reliability assesses the consistency of results at different timepoints when underlying conditions remain the same. Test–retest reliability was assessed by calculating the ICC based on two successive SV95C recording periods 1 month apart (N = 52 patients with DMD from studies CT-A, NHS-C, and in-clinic). Average SV95C measures from the first period were compared with those from the second using a two-way random effects model to calculate absolute agreement. SV95C variability and the influence of time of recording were assessed in patients and controls.
ICCs stratified by age group were calculated based on measures performed 15 days apart in two successive recording periods (N = 103 patients from CT-A, NHS-C, in-clinic, and CT-B).
Known-groups validity
Known-groups validity is the ability of a measure to distinguish groups with known relevant differences. The known-groups validity of SV95C was analyzed by comparing patients with DMD aged 5 − 14 years (N = 125) with controls aged 6 − 14 years (N = 66). Similar comparisons were made for the 6MWD and 4SC. Controls did not perform the NSAA. Independent-Samples Mann–Whitney U Tests were used for two-sample comparisons, and Independent-Samples Kruskal–Wallis Tests for comparisons involving > 2 samples.
Responsiveness
Responsiveness is the ability of an assessment to detect change over time. Responsiveness was assessed in a subset of patients with DMD from three natural history studies and two clinical trials without observed treatment efficacy, enabling comparisons with 6MWD and NSAA up to 12 months post-baseline (minimum participant numbers were N = 43 [3 months] and N = 15 [12 months]). Responsiveness was assessed based on natural change in SV95C at months 3 (N = 81), 6 (N = 59), 9 (N = 39), and 12 (N = 28). Patients were on a stable corticosteroid regimen or had initiated corticosteroids ≥ 6 months before study initiation. SV95C’s responsiveness to the treatment effect of corticosteroid initiation was assessed at months 3 (N = 11), 6 (N = 7), 9 (N = 11), and 12 (N = 5). SRMs were calculated as the mean of the change divided by the standard deviation of the change.
Meaningful change
Anchor-based, within-patient MCTs for SV95C in patients with DMD were analyzed based on the CGI-C (N = 12) and PODCI (N = 15) “transfers and basic mobility” subdomain (Supplementary Methods B).
The anchor-based, within-patient MCT was also assessed using 48-week changes in reference functional tests, including the 6MWD (N = 13) and NSAA (N = 12), based on the MDC at the 80% CL (2.78 points for NSAA and 36.3 m for 6MWD).
Distribution-based meaningful change was analyzed via the standard error of measurement and MDC (Supplementary Methods C).
Qualitative evidence
During initial SV95C development, patients, caregivers, patient advocacy groups, experts, and industry representatives were consulted about the relevance of stride velocity in DMD. Feedback was obtained from expert panels and initial industry users of the wearable.
Qualitative evidence for the clinical relevance of SV95C was explored using data derived from the following sources (additional details are included in Supplementary Methods D): (1) an online survey of patients with neuromuscular diseases and their caregivers, in which 92 of the 549 responders (including 14 patients and 78 caregivers) were living with or caring for someone with DMD (characteristics of survey respondents are available in Supplementary Table S4, and patient survey questions are presented in Supplementary Table S5); (2) an online survey gathering feedback on ActiMyo experiences from 52 worldwide healthcare professionals, including physiotherapists (57%), study coordinators/nurses/managers (34%), and physicians (9%) (see survey questions in Supplementary Table S6); (3) eight solicited letters of support from European neurologists and physiotherapists; (4) comments received from nonprofit organizations, industry representatives, and the broader scientific community during the public consultation for the previous SV95C secondary endpoint EMA qualification; and (5) responses from live polling conducted during the 2018 Parent Project Muscular Dystrophy annual congress, as well as support letters from the patient association.
Supplementary Information
Acknowledgements
Medical writing and editorial support were provided by Jen Ciarochi, PhD, of Nucleus Global in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022) and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Author contributions
L.S., P.S., A.T., and D.E. were responsible for the study concept and design, acquisition, analysis, or interpretation of data, and drafting of the manuscript. A.T. and D.E. were responsible for the statistical analysis. All authors were responsible for critical revision of the manuscript for important intellectual content.
Funding
The development of this article was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Data availability
The data that support the findings of this study are housed with the sponsors of the individual constituent studies and so are not publicly available. Data are available upon reasonable request from Damien Eggenspieler (damien.eggenspieler@sysnav.fr).
Declarations
Competing interests
L.S. is a member of scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, Biophytis, Cytokinetics, Dynacure, F. Hoffmann-La Roche Ltd, GeneTx Biotherapeutics, REGENXBIO, Santhera Pharmaceuticals, and Sarepta Therapeutics, Inc., has consulted for Pfizer and Affinia, conducts research funded by Novartis Gene Therapies (formerly AveXis), Biogen, and F. Hoffmann-La Roche Ltd, holds part of the patent WO2017129890A1 with no financial interest, and has provided consultancy services to SYSNAV. P.S. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. M.P. reports no disclosures relevant to the manuscript. A.M. reports no disclosures relevant to the manuscript. N.B. reports no disclosures relevant to the manuscript. V.A.S. provides intellectual consultancies and teaching activities for Biogen, F. Hoffmann-La Roche Ltd, Novartis, Lupin, Dyne Therapeutics, and PTC Therapeutics. C.V. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, Italfarmaco, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. U.S.S. is a member of scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Pfizer, Santhera Pharmaceuticals, Sarepta Therapeutics, Inc., Italfarmaco, and PTC Therapeutics, and has received honoraria for invited talks or chair positions in scientific symposia from Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Pfizer, Santhera Pharmaceuticals, Sarepta Therapeutics, Inc., Italfarmaco, and PTC Therapeutics. M.S. has provided consultancy services for and received honoraria (as a member of scientific advisory boards) from Biogen, F. Hoffmann-La Roche Ltd, and Novartis Gene Therapies (formerly AveXis). A.M.S. reports no disclosures relevant to the manuscript. S.C.P. reports participation in scientific advisory boards for EspeRare Foundation, Wave Life Sciences Ltd, Argenx, and Sarepta Therapeutics, Inc., and has provided consultancy service for Alia Therapeutics and LSC Lifesciences. M.T. has participated in scientific advisory boards for Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, and Sarepta Therapeutics, Inc., and has received honoraria for invited lectures from Biogen, Sarepta Therapeutics, Inc., and PTC Therapeutics. A.N. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, Italfarmaco, Pfizer, Dyne Therapeutics, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis) and Biogen. P.F. reports no disclosures relevant to the manuscript. T. S. is an employee of Sarepta Therapeutics, Inc. and has stock and stock options. R.D.D. is Head of Clinical Development at Solid Biosciences, was previously employed at F. Hoffmann-La Roche Ltd, Santhera Pharmaceuticals, and Novartis, and has stock in Solid Biosciences and F. Hoffmann-La Roche Ltd. N.G. reports activities as a Data and Safety Monitoring Board member for Pfizer, Antisense Therapeutics, Wave Life Sciences Ltd, and Genethon. E.M. has served on clinical steering committees and/or as a consultant and received compensation from Italfarmaco, PTC Therapeutics, Sarepta Therapeutics, Inc., Santhera Pharmaceuticals, Pfizer Inc., F. Hoffmann-La Roche Ltd, Wave Life Sciences, NS Pharma, and Dyne Therapeutics, and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. V.S. has served on scientific advisory boards for Astellas Gene Therapies, Biogen, Edgewise Therapeutics, Ipsen, Kate Therapeutics, ML Bio Solutions, Novartis Gene Therapies, PepGen, F. Hoffmann-La Roche Ltd, Sanofi, Sarepta Therapeutics, Inc., Vertex Pharmaceuticals, and Wave Therapeutics, has received speaker honoraria from Pfizer, F. Hoffmann-La Roche Ltd, Sanofi, and Sarepta Therapeutics, Inc., has received grants for clinical research from Sarepta Therapeutics, Inc. and Sanofi, and has received support from the NIHR Newcastle Biomedical Research Centre. M.G.O. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. J.B. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. F.M. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Italfarmaco, Pfizer, Dyne Therapeutics, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. A.T. has nothing to disclose other than his employment at SYSNAV, a company that collaborated with the Institute of Myology to create ActiMyo®. M.A. was an employee of SYSNAV at the time that this manuscript was developed. D.E. has nothing to disclose other than his employment at SYSNAV, a company that collaborated with the Institute of Myology to create ActiMyo®.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80177-9.
References
- 1.Crisafulli, S. et al. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet. J. Rare Dis.15, 141. 10.1186/s13023-020-01430-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markati, T. et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol.21, 814–829. 10.1016/S1474-4422(22)00125-9 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Kempf, L., Goldsmith, J. C. & Temple, R. Challenges of developing and conducting clinical trials in rare disorders. Am. J. Med. Genet. A.176, 773–783. 10.1002/ajmg.a.38413 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Pizzamiglio, C., Vernon, H. J., Hanna, M. G. & Pitceathly, R. D. S. Designing clinical trials for rare diseases: unique challenges and opportunities. Nat. Rev. Methods Primers10.1038/s43586-022-00100-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Qualification opinion for stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne muscular dystrophy studies, https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-primary-endpoint-studies-ambulatory-duchenne_en.pdf (2023).
- 6.Mittermaier, M., Venkatesh, K. P. & Kvedar, J. C. Digital health technology in clinical trials. NPJ Digit. Med.6, 88. 10.1038/s41746-023-00841-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratitch, B. et al. Clinical validation of novel digital measures: statistical methods for reliability evaluation. Digit. Biomark.7, 74–91. 10.1159/000531054 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Servais, L., Yen, K., Guridi, M. & Lukawy, J. Stride velocity 95th centile: Insights into gaining regulatory qualification of the first wearable-derived digital endpoint for use in Duchenne muscular dystrophy trials. J. Neuromuscul. Dis.9, 335–346. 10.3233/JND-210743 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izmailova, E. S., Demanuele, C. & McCarthy, M. Digital health technology derived measures: Biomarkers or clinical outcome assessments?. Clin. Transl. Sci.16, 1113–1120. 10.1111/cts.13529 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Qualification opinion on stride velocity 95th centile as a secondary endpoint in Duchenne Muscular Dystrophy measured by a valid and suitable wearable device, https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-secondary-endpoint-duchenne-muscular-dystrophy_en.pdf (2018).
- 11.Haberkamp, M. et al. European regulators’ views on a wearable-derived performance measurement of ambulation for Duchenne muscular dystrophy regulatory trials. Neuromuscul. Disord.29, 514–516. 10.1016/j.nmd.2019.06.003 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Servais, L. et al. First regulatory qualification of a digital primary endpoint to measure treatment efficacy in DMD. Nat. Med.29, 2391–2392. 10.1038/s41591-023-02459-5 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Mayhew, A. G. et al. Functional outcome measures in young, steroid-naïve boys with Duchenne muscular dystrophy. Neuromuscul. Disord.32, 460–467. 10.1016/j.nmd.2022.02.012 (2022). [DOI] [PubMed] [Google Scholar]
- 14.McDonald, C. M. et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve.48, 357–368. 10.1002/mus.23905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emery, N., Strachan, K., Kulshrestha, R., Kuiper, J. H. & Willis, T. Evaluating the feasibility and reliability of remotely delivering and scoring the north star ambulatory assessment in ambulant patients with duchenne muscular dystrophy. Children (Basel).9, 728. 10.3390/children9050728 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott, E. et al. Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy. Physiother. Res. Int.17, 101–109. 10.1002/pri.520 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Henricson, E. et al. The 6-minute walk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr.10.1371/currents.md.9e17658b007eb79fcd6f723089f79e06 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercuri, E. et al. Revised north star ambulatory assessment for young boys with duchenne muscular dystrophy. PLoS One.11, e0160195. 10.1371/journal.pone.0160195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gidaro, T. et al. Home-based gait analysis as an exploratory endpoint during a multicenter phase 1 trial in limb girdle muscular dystrophy type R2 and facioscapulohumeral muscular dystrophy. Muscle Nerve.65, 237–242. 10.1002/mus.27446 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Annoussamy, M. et al. Natural history of Type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann. Clin. Transl. Neurol.8, 359–373. 10.1002/acn3.51281 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Moing, A. G. et al. A movement monitor based on magneto-inertial sensors for non-ambulant patients with duchenne muscular dystrophy: a pilot study in controlled environment. PLoS One.11, e0156696. 10.1371/journal.pone.0156696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duis, J. et al. Quantitative measures of motor development in Angelman syndrome. Am. J. Med. Genet. A.191, 1711–1721. 10.1002/ajmg.a.63192 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricotti, V. et al. Wearable full-body motion tracking of activities of daily living predicts disease trajectory in Duchenne muscular dystrophy. Nat. Med.29, 95–103. 10.1038/s41591-022-02045-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poleur, M. et al. Normative data on spontaneous stride velocity, stride length, and walking activity in a non-controlled environment. Orphanet. J. Rare Dis.16, 318. 10.1186/s13023-021-01956-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servais, L. et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucl. Acid Ther.32, 29–39. 10.1089/nat.2021.0043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabbia, M. et al. Stride velocity 95th centile detects decline in ambulatory function over shorter intervals than the 6-minute walk test or North Star ambulatory assessment in duchenne muscular dystrophy. J. Neuromuscul. Dis.11, 701–714. 10.3233/JND-230188 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldsack, J. C. et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for biometric monitoring technologies (BioMeTs). NPJ Digit Med.3, 55. 10.1038/s41746-020-0260-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.OptiTrack, https://optitrack.com/ (2024).
- 29.Goemans, N. et al. Prognostic factors for changes in the timed 4-stair climb in patients with Duchenne muscular dystrophy, and implications for measuring drug efficacy: A multi-institutional collaboration. PLoS One.15, e0232870. 10.1371/journal.pone.0232870 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chetta, A. et al. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir. Med.100, 1573–1578. 10.1016/j.rmed.2006.01.001 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are housed with the sponsors of the individual constituent studies and so are not publicly available. Data are available upon reasonable request from Damien Eggenspieler (damien.eggenspieler@sysnav.fr).