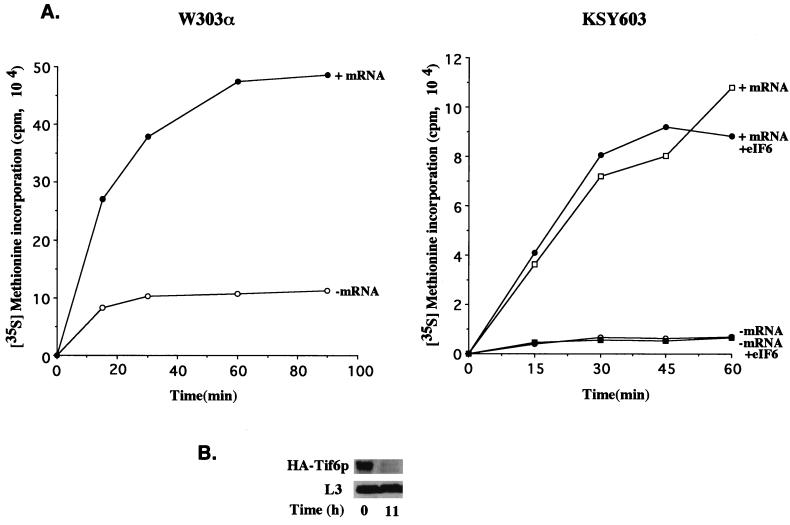
FIG. 7.
In vitro translation of total yeast poly(A)+ RNA in eIF6-depleted cell extracts. (A) Exponentially growing cultures of KSY603 or W303α cells in YPGal medium were harvested and suspended in glucose (YPD) medium such that the initial A600 was about 0.03 U. At about 11 h after the shift, the cells were harvested and translation cell extracts were prepared, incubated, and analyzed for [35S]methionine incorporation into proteins as described in Materials and Methods. Where indicated, 5 μg of total yeast poly(A)+ RNA (mRNA) and 0.5 μg of recombinant yeast eIF6 were added to 50 μl of reaction mixtures containing 25 μCi of [35S]methionine and all the other components required for translation including micrococcal nuclease-treated cell extracts containing approximately 150 μg of proteins. Aliquots (8 μl) from 50-μl reaction mixtures were withdrawn at the indicated times and analyzed for [35S]methionine incorporation into proteins as described in Materials and Methods. (B) At the indicated times following the shift from YPGal to YPD medium, cell lysates were prepared and 200 μg of protein from each lysate was subjected to Western blot analysis with either anti-HA monoclonal antibodies or anti-L3 antibodies as probes. It should be noted that a relatively large amount of cell lysates was analyzed in the Western blot to ensure that the lysates had no detectable levels of eIF6. Under these conditions, the level of the 60S ribosomal protein L3 was also decreased. However, no change in the level of L3 in cell lysates between 0 and 11 h was apparent in the immunoblot analysis, presumably because the amount of L3 in cell lysates analyzed was still large.