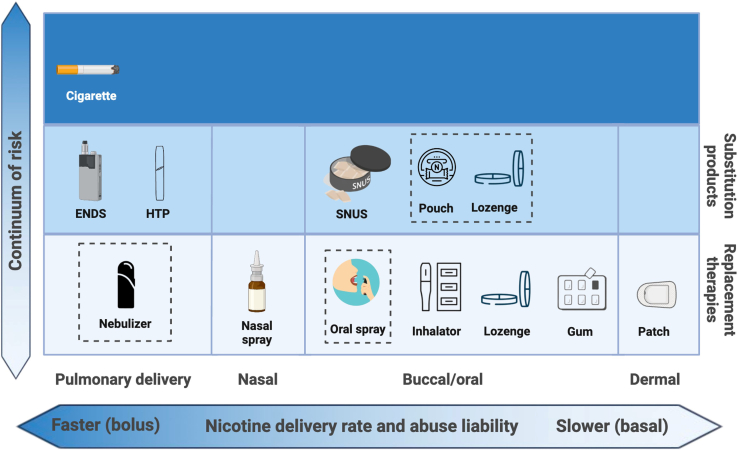
Figure 2.
Nicotine delivery products for stopping smoking
Cigarettes and nicotine delivery products span a continuum of risk (X axis). They also vary in route of administration and nicotine delivery rates (Y axis), which impact abuse liability and efficacy in stopping smoking. Nicotine replacement therapies meet medicinal regulatory standards of safety and are lowest on the harm continuum of nicotine products. NRTs approved in the US include patches, gum, lozenges, inhalators, and nasal inhaler. Pipeline products (dotted lines) include oral spray, nebulizer, and medicinal ENDS (in England). Nicotine substitution products are intermediate in the continuum of risk. Products which the FDA-CTP (Center for Tobacco Products) has scientifically reviewed and designated as meeting the APPH (appropriate for the protection of the public health) marketing authorization regulatory standard include ENDS, HTP, and SNUS products. Nicotine pouches and lozenges are under FDA review. Not shown: very low nicotine content cigarettes (VLNCs), which provide reduced nicotine exposure vs. conventional cigarettes, although with similarly toxic smoke. Created in BioRender. Mangione, M. (2024) BioRender.com/q50z218.