Abstract
Syphilis, caused by the spirochete Treponema pallidum, is a sexually transmitted infection (STI) that has seen a resurgence worldwide, particularly among populations at a higher risk of co-infection with human immunodeficiency virus (HIV). The disease typically progresses through distinct stages: primary, secondary, latent, and tertiary, each with specific clinical manifestations. Secondary syphilis is characterized by systemic involvement and various mucocutaneous symptoms, including a maculopapular rash that frequently involves the palms and soles along with fever, lymphadenopathy, and mucous membrane lesions. However, in patients with HIV co-infection, syphilis may present atypically. The immunosuppression caused by HIV can lead to more severe, atypical, and persistent manifestations of secondary syphilis. Furthermore, the cutaneous features may deviate from the classic presentation, making diagnosis challenging. We report the case of a male in his third decade of life, recently diagnosed with HIV, who presented with diffuse hyperpigmented dermatosis. The unusual presentation, including well-defined brown macules with a generalized distribution, initially raised suspicion for Kaposi’s sarcoma (KS), a frequent cutaneous malignancy seen in HIV patients. Skin biopsy showed a dense perivascular and interstitial inflammatory infiltrate with marked endothelial swelling and vascular proliferation. Immunohistochemistry confirmed the presence of Treponema spirochetes, and a positive Venereal Disease Research Laboratory (VDRL) test further supported the diagnosis of secondary syphilis in the context of HIV. Our case underscores the importance of considering secondary syphilis in the differential diagnosis in cases of generalized hyperpigmented dermatosis in newly diagnosed HIV patients, where common conditions such as Kaposi’s sarcoma may obscure the underlying etiology.
Keywords: aids (acquired immunodeficiency syndrome), dermatosis, hiv aids, secondaryism, syphilis
Introduction
Syphilis, caused by the spirochete Treponema pallidum, is a sexually transmitted infection with systemic implications, primarily affecting the skin and mucous membranes. It progresses through three distinct stages: primary, secondary, and tertiary. Secondary syphilis, in particular, presents a broad spectrum of clinical manifestations. The characteristic rash can appear as macules or papules, often desquamating, typically involving the palms and soles, and is generally non-pruritic [1,2].
Other common features include oral ulcers, mucous patches, diffuse alopecia, generalized lymphadenopathy, and in moist areas such as the axillae and perineum, soft, raised lesions known as condylomata lata. Secondary syphilis may also involve other organ systems, causing hepatitis, nephritis, and central nervous system involvement [3].
In individuals living with HIV, secondary syphilis is more prevalent and frequently presents with atypical and more severe manifestations, necessitating increased clinical vigilance [4]. Cutaneous manifestations are among the most common presentations and can exhibit unusual characteristics, complicating diagnosis [5-9].
Here, we present the case of a male in his third decade of life, without a known history of HIV, who presented with a generalized dermatosis of brown macules. Our case highlights the critical need to consider secondary syphilis in the differential diagnosis of generalized hyperpigmented dermatosis in newly diagnosed HIV patients, where common conditions like Kaposi’s sarcoma can obscure the true etiology.
Case presentation
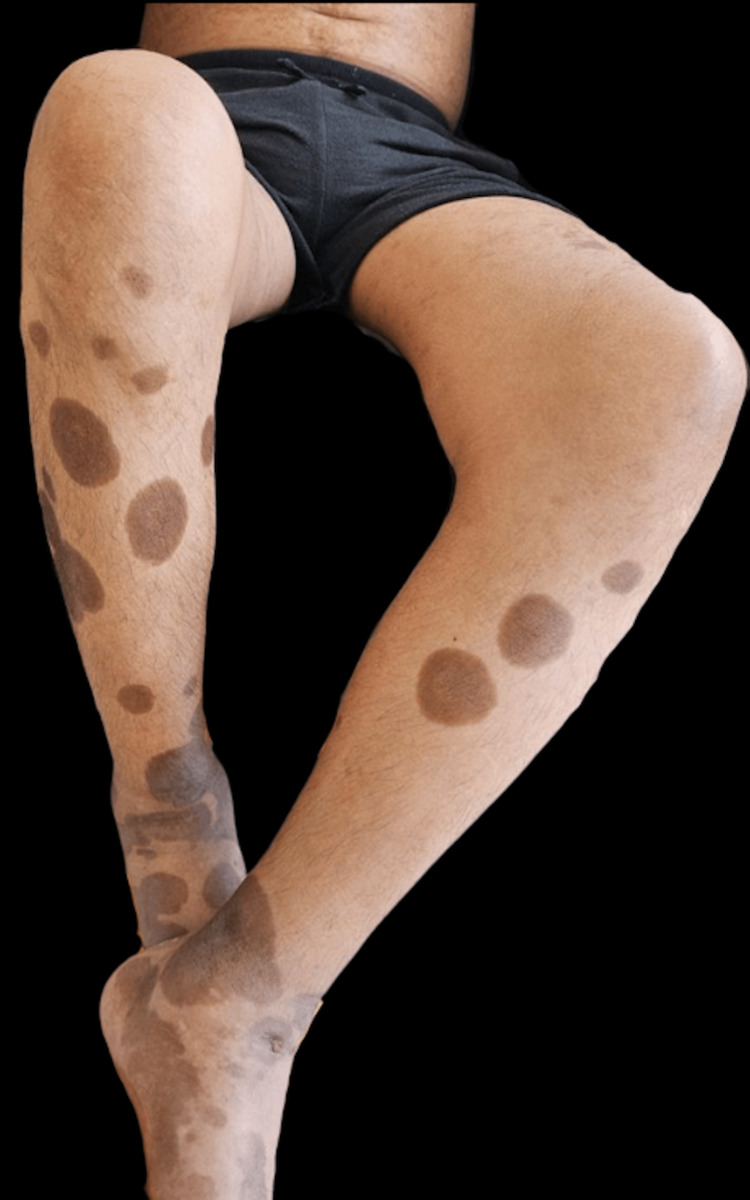
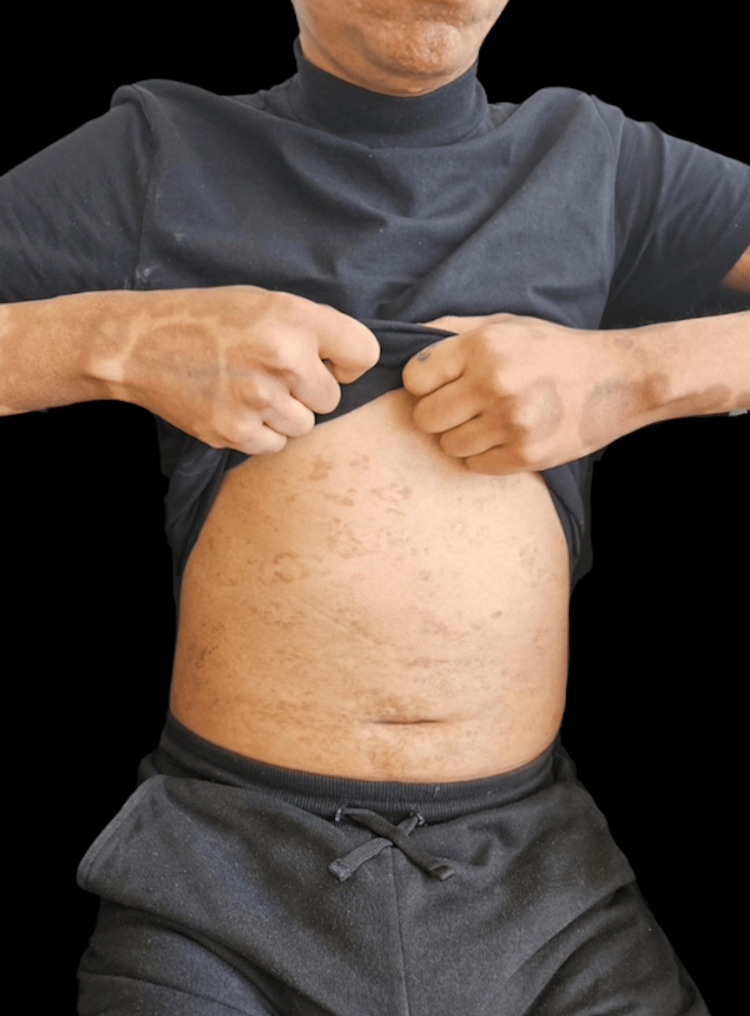
A 37-year-old male with no known history of HIV or other comorbidities, and a history of male-to-male sexual contact, presented to the outpatient dermatology clinic with a generalized dermatosis characterized by symmetrical, well-defined, hyperpigmented brown macules on his hands and palms, which later spread to his lower extremities, trunk, and face (Figures 1, 2). We performed a skin biopsy; however, the patient was lost to follow-up.
Figure 1. Asymmetrical hyperpigmented dermatosis on the lower extremities.
Asymmetrical, well-defined brown macules are visible on both lower extremities.
Figure 2. Hyperpigmented dermatosis on the trunk.
Asymmetrical, well-defined, hyperpigmented brown macules on the trunk
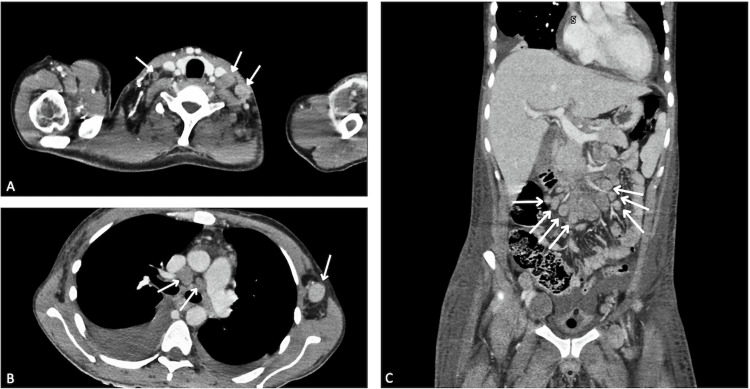
Five months later, the patient returned to the emergency department with unintentional weight loss exceeding 15% of his body weight, along with persistent fatigue and generalized weakness. Physical examination revealed mobile, non-tender cervical and axillary lymphadenopathy. Vital signs upon arrival were: blood pressure 120/70 mmHg, heart rate 127 bpm, respiratory rate 18 breaths per minute, and temperature 36.7 °C. Initial laboratory tests are detailed in Table 1. We performed a contrast-enhanced computed tomography (CT) scan of the chest and abdomen due to the presence of lymphadenopathy, which revealed cervical, mediastinal, axillary, retroperitoneal, and inguinal lymphadenopathy, as well as bilateral pleural effusion with right-sided predominance (Figure 3).
Table 1. Initial laboratory results for the patient at admission.
| Category | Result | Normal range | ||
| Blood Counts | ||||
| Hemoglobin (mg/dL) | 6.65 | 12.1-15.1 | ||
| Hematocrit (%) | 22.1 | 36.1-44.3 | ||
| White Blood Count (K/mcL) | 14.2 | 12.9 4.5-11.0 | ||
| Neutrophils (K/mcL) | 10.9 | 11.5 1.8-7.8 | ||
| Lymphocytes (K/mcL) | 1.9 | 0.6-3.4 | ||
| Metabolic Panel | ||||
| Platelets (K/mcL) | 45.3 | 150-450 | ||
| Creatinine (mg/dL) | 0.6 | 0.59-1.04 | ||
| Blood Urea Nitrogen (mg/dL) | 13 | 35 7-20 | ||
| Glucose (mg/dL) | 116 | 74-106 | ||
| Albumin (g/dL) | 1.9 | 3.4-5.4 | ||
| Inflammatory Markers | ||||
| Erythrocyte Sedimentation Rate (mm/hr) | 32 | 0-29 | ||
| C-Reactive Protein (mg/dL) | 9.9 | <1.0 | ||
| Hepatic Function | ||||
| Total Bilirubin (mg/dL) | 0.6 | 0.2-1.0 | ||
| Aspartate Aminotransferase (UI/L) | 20 | 5-40 | ||
| Alanine Aminotransferase (UI/L) | 6 | 7-56 | ||
| Lactate Dehydrogenase (UI/L) | 275 | 91-180 | ||
Figure 3. Contrast-enhanced computed tomography (CT) scan of the chest and abdomen.
Contrast-enhanced CT scan showing A and B: axial sections
Arrows: cervical and mediastinal/axillary lymphadenopathy involvement. C: coronal section. Arrows: retroperitoneal lymphadenopathy
HIV testing was performed, revealing positive HIV-1 and HIV-2 antibody tests, with a subsequent viral load of 38,448 copies/mL and a CD4 count of 399 cells/µL. Further testing for opportunistic infections, including serum Cryptococcal antigen and an interferon-gamma release assay for tuberculosis, was conducted, both of which were negative. The serum Venereal Disease Research Laboratory (VDRL) test for syphilis was positive at a titer of 1:32.
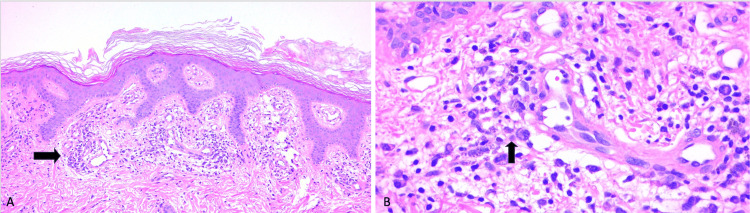
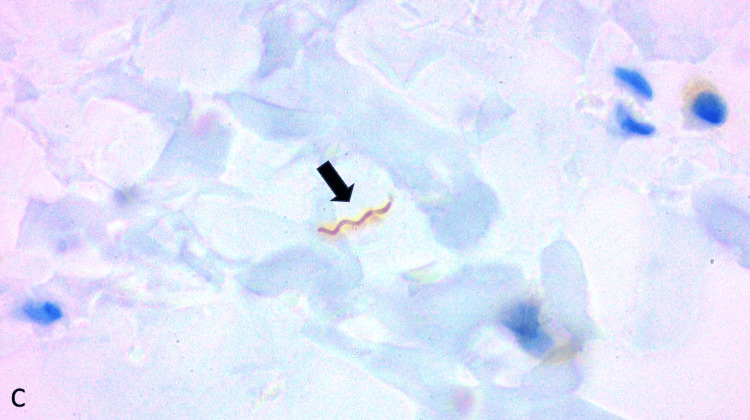
The skin biopsy revealed a dense perivascular and interstitial inflammatory infiltrate composed of lymphocytes, plasma cells, and histiocytes (Figure 4). There was marked endothelial swelling and prominent vascular proliferation. Warthin-Starry staining was negative; therefore, we performed immunohistochemical staining for Treponema, which was positive for the presence of spirochetes (Figure 5), confirming a diagnosis of secondary syphilis, particularly concerning in the context of concurrent HIV infection.
Figure 4. Histological section of the skin from the trunk.
A) Arrow: perivascular inflammatory process in the superficial dermis, magnification 20x; B) Arrow: lymphoplasmacytic infiltrate around blood vessels, magnification 40x
Figure 5. Immunohistochemical staining showing positive reactivity for Treponema.
Arrow: Positive Treponema stain for structures compatible with spirochetes, magnification 100x
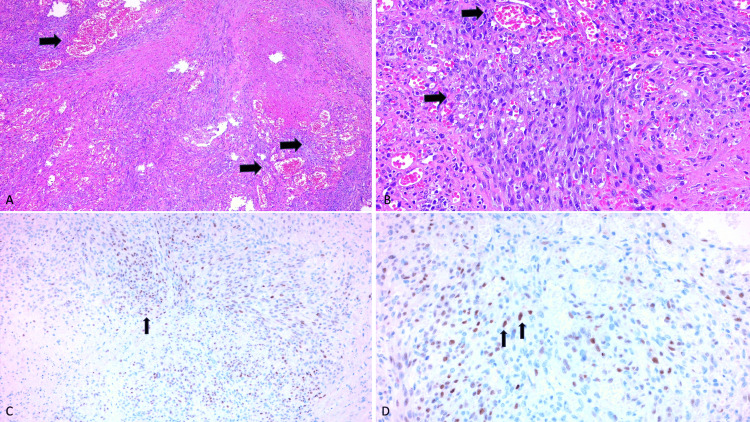
Given the significant lymphadenopathy, a cervical lymph node biopsy was performed. Histopathological examination demonstrated a dense proliferation of spindle cells with irregular, slit-like vascular spaces, extravasation of erythrocytes, hemosiderin deposits, and a mixed inflammatory infiltrate, consistent with Kaposi sarcoma (Figures 6A, 6B). Immunohistochemical staining for human herpesvirus 8 (HHV-8) was positive, confirming the diagnosis (Figures 6C, 6D).
Figure 6. Histological section of a cervical lymph node.
A and B) Lymph node biopsy showing: Arrows: Proliferation of dilated vascular channels with round spindle cells, magnification 20x and 40x, respectively. C and D) Immunohistochemistry staining for HHV8 showing: Arrows: granular nuclear positivity, magnification 20x and 40x, respectively.
The patient was initiated on prophylactic treatment with trimethoprim/sulfamethoxazole and benzathine penicillin G for secondary syphilis (2.4 million IU, single dose). During his hospitalization, the patient developed episodes of hematochezia, prompting a colonoscopy. The examination revealed multiple violaceous nodules and plaques throughout the colonic mucosa, predominantly in the descending and sigmoid colon. The lesions varied in size, with some appearing as polypoids and others as flat plaques. Several areas showed friable mucosa with scattered ulcerations, consistent with extensive involvement by Kaposi sarcoma, likely contributing to the ongoing bleeding.
Despite initial management, the patient’s clinical condition deteriorated rapidly, and he developed signs of hypovolemic shock, including hypotension and tachycardia, due to continued gastrointestinal bleeding. Intensive resuscitation efforts, including fluid and blood product replacement, were initiated, but unfortunately, the bleeding persisted and the patient did not respond to treatment. His hemodynamic status continued to worsen, and despite exhaustive efforts, he ultimately passed away due to the severity of his condition.
Discussion
The clinical presentation of syphilis in patients living with HIV may be atypical due to the state of immunosuppression. We present a patient in whom the initial suspected diagnosis was Kaposi's sarcoma (KS) due to the characteristics of the dermatosis, which was marked by hyperpigmented, brown-colored spots with hyperkeratosis and a generalized distribution, in contrast to the classic presentation of secondary syphilis (a pink-reddish maculopapular rash). Moreover, considering the general symptoms, the lymph node biopsy result, and the nature of the dermatosis, the clinical picture was initially consistent with KS.
However, based on the skin biopsy and VDRL results, cutaneous KS was ruled out. Unlike the majority of reported cases of secondary syphilis in patients with HIV co-infection [5,10-12], our patient did not present with nodular or ulcerated lesions, as previously reported. To our knowledge, there are no other reported cases where secondary syphilis mimicked macular KS. While there have been cases [13-15] of secondary syphilis mimicking KS, their clinical presentations included indurated nodular lesions, similar to other cases of secondary syphilis in HIV co-infection. Furthermore, it is important to highlight that in our patient, secondary syphilis was the primary manifestation of AIDS.
Doung et al. reported the case of an HIV-positive patient with a clinical scenario similar to ours, including cutaneous manifestations, cervical lymphadenopathy, and intestinal bleeding. They described macular lesions with scaling on both hands and isolated black-violaceous papules with necrotic centers on the abdomen, measuring less than 5 cm [9]. However, unlike our patient, these were not generalized lesions, and the cutaneous manifestation was not the main reason for consultation.
A case report published by Gori et al. closely resembles ours, describing a 38-year-old patient with no significant medical history, diagnosed with secondary syphilis co-infected with HIV and Kaposi's sarcoma [13]. Although the lesions differed from those of our patient, the location was similar. Their patient presented with smooth, fixed, reddish-purple papulonodular lesions on the face, arms, trunk, and legs, unlike the hyperpigmented macules observed in our case, which included the hands and palms. Despite a similar diagnostic workup, our patient only presented with fatigue and generalized weakness, without symptoms such as night sweats, abdominal pain, or bloody diarrhea. The VDRL titer in Gori’s case was 1:64, and the CD4+ cell count was 525 cells/µL.
In our case, secondary syphilis was confirmed through biopsy and VDRL results. The patient received appropriate treatment for syphilis, though antiretroviral therapy was delayed. This case emphasizes the importance of conducting a thorough differential diagnosis in HIV patients with dermatitis. Although Kaposi's sarcoma is a common cause of skin lesions in these patients, other etiologies, such as sexually transmitted infections, should not be ruled out.
The management of secondary syphilis in HIV patients requires close monitoring and adequate treatment to prevent long-term complications. Penicillin remains the treatment of choice; however, in patients with HIV infection, higher doses or longer treatments may be necessary due to suboptimal responses associated with probable resistance and immunosuppression, leading to an increased bacterial load [5]. In our patient, it was not possible to assess the response to a single dose of benzathine G penicillin as recommended by European Guidelines [16]; however, an alternative treatment regimen has proven effective in other cases of malignant syphilis in immunosuppressed patients. For example, Lopez et al. reported a 29-year-old woman with a history of hypothyroidism and malnutrition who presented with malignant syphilis. In this case, weekly doses of benzathine G penicillin for three weeks resulted in the resolution of cutaneous lesions, with serological improvement observed after three months [17].
Conclusions
This case highlights the need for a comprehensive diagnostic approach in patients with HIV and dermatosis. It underscores the importance of conducting a thorough differential diagnosis in HIV patients presenting with skin lesions. While Kaposi's sarcoma is a common cause of skin lesions in these patients, other etiologies, such as sexually transmitted infections, should not be overlooked. The coexistence of secondary syphilis and HIV illustrates the complexity of managing these co-infections and emphasizes the need for timely and accurate diagnosis to provide targeted treatment and improve patient outcomes.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Victor D. Acuña Rocha, Ricardo A. López Pérez, Victoria Sauza Gonzalez, Anette Fischer Rouyer, Ashley Lilian Villapudua Torres, Rodolfo Franco Márquez
Acquisition, analysis, or interpretation of data: Victor D. Acuña Rocha, Ricardo A. López Pérez, Victoria Sauza Gonzalez, Anette Fischer Rouyer, Ashley Lilian Villapudua Torres, Rodolfo Franco Márquez
Drafting of the manuscript: Victor D. Acuña Rocha, Ricardo A. López Pérez, Victoria Sauza Gonzalez, Anette Fischer Rouyer, Ashley Lilian Villapudua Torres, Rodolfo Franco Márquez
Critical review of the manuscript for important intellectual content: Victor D. Acuña Rocha, Ricardo A. López Pérez, Victoria Sauza Gonzalez, Anette Fischer Rouyer, Ashley Lilian Villapudua Torres, Rodolfo Franco Márquez
Supervision: Victor D. Acuña Rocha, Ricardo A. López Pérez, Ashley Lilian Villapudua Torres, Rodolfo Franco Márquez
References
- 1.Syphilis. Peeling RW, Mabey D, Chen XS, et al. Lancet. 2023;402:336–346. doi: 10.1016/S0140-6736(22)02348-0. [DOI] [PubMed] [Google Scholar]
- 2.Syphilis: a modern resurgence. Ramchandani MS, Cannon CA, Marra CM. Infect Dis Clin North Am. 2023;37:195–222. doi: 10.1016/j.idc.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Syphilis. Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS. Nat Rev Dis Primers. 2017;3:17073. doi: 10.1038/nrdp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atypical secondary syphilis presentation in a patient with human immunodeficiency virus infection: a case report. Yancheva N, Petrova E, Tchervenyakova T. J Med Case Rep. 2019;13:360. doi: 10.1186/s13256-019-2291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nodular secondary syphilis in three HIV-positive patients: a case series. Wang CJ, Leavens J, Thorpe J, Crew A, Kim G, Ahronowitz I, Worswick S. Int J STD AIDS. 2020;31:1004–1007. doi: 10.1177/0956462420933787. [DOI] [PubMed] [Google Scholar]
- 6.Erythema multiforme triggered by Treponema pallidum infection in an HIV-infected patient. Zanella LG, Sampaio ÉF, Lellis RF. Int J STD AIDS. 2018;29:99–102. doi: 10.1177/0956462417726203. [DOI] [PubMed] [Google Scholar]
- 7.Diphasic fever with generalised rash including palm and sole: secondary syphilis and HIV coinfection. Yoshikawa H, Shikino K, Hoshina Y, Ikusaka M. BMJ Case Rep. 2020;13:0. doi: 10.1136/bcr-2020-238013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secondary syphilis mimicking tinea cruris in an HIV infected patient: a case report. Yuylana C, Iswanty M, Paturusi II. Pan Afr Med J. 2021;38:133. doi: 10.11604/pamj.2021.38.133.27922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secondary syphilis mimicking Kaposi sarcoma in an HIV patient. Duong TA, Valeyrie-Allanore L, Grange P, et al. Eur J Dermatol. 2019;29:432–433. doi: 10.1684/ejd.2012.1905. [DOI] [PubMed] [Google Scholar]
- 10.Generalized asymptomatic nodulo-ulcerative lesions without systemic symptoms in a secondary syphilis patient co-infected with HIV. Maharani RH, Nugraha T, Sutedja E, Ruchiatan K, Usman HA, Achdiat PA. https://pubmed.ncbi.nlm.nih.gov/38144158/ Clin Cosmet Investig Dermatol. 2023;16:3645–3650. doi: 10.2147/CCID.S445155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malignant syphilis: an early feature of underlying HIV infection in an MSM patient. Alfieri A, Irmawati YE, Pudjiati SR. Germs. 2023;13:168–171. doi: 10.18683/germs.2023.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secondary syphilis as an initial presentation of HIV. Khan M, Kaur T, Phan T, Yassin M. Proc (Bayl Univ Med Cent) 2022;35:66–67. doi: 10.1080/08998280.2021.1953952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secondary syphilis mimicking Kaposi sarcoma in an HIV patient. Gori A, Maio V, Grazzini M, et al. Eur J Dermatol. 2013;23:120–121. doi: 10.1684/ejd.2012.1905. [DOI] [PubMed] [Google Scholar]
- 14.Malignant syphilis requiring differentiation from Kaposi's sarcoma. Tokano M, Tarumoto N, Imai K, et al. IDCases. 2024;36:0. doi: 10.1016/j.idcr.2024.e01943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Yayli S, della Torre R, Hegyi I, Schneiter T, Beltraminelli H, Borradori L, Fux C. Int J Dermatol. 2014;53:0–3. doi: 10.1111/j.1365-4632.2012.05598.x. [DOI] [PubMed] [Google Scholar]
- 16.2020 European guideline on the management of syphilis. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. J Eur Acad Dermatol Venereol. 2021;35:574–588. doi: 10.1111/jdv.16946. [DOI] [PubMed] [Google Scholar]
- 17.Malignant syphilis: a rare case of early secondary syphilis in an immunocompetent patient. Sá Lopes R, Monteiro AS, Saez R, Candeias C, Mendonça C. Eur J Case Rep Intern Med. 2023;10:3721. doi: 10.12890/2023_003712. [DOI] [PMC free article] [PubMed] [Google Scholar]