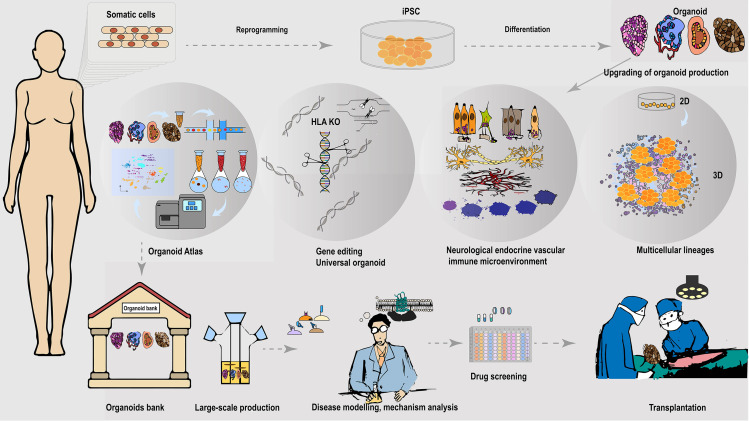
Figure 2. Universally compatible iPSC-organoid biobanking and applications.
iPSC could be reprogrammed from a patient’s somatic cells and used as a starting source for producing patient-derived multiple desired organoids. Advanced 3D organoid culture system contains multiple supportive cell populations such as stromal cells, endothelial cells, as well as the neuroendocrine and immune system, allowing for a closer approximation to in vivo organs. The inclusion of multiple supportive cell populations contributes to the construction of disease models and the development of pharmaceutical products at this stage. The automated culture system provides the possibility to overcome the variable errors caused by manual inconsistency, and scale-up of support organoid production, and is expected to provide a solution to the difficult breakthrough of large-scale expansion of standardized organoids at the GMP level. In addition, the use of gene-editing tools to knock out immune response antigens such as HLA is expected to generate universally compatible iPSC-organoids ideal for allogeneic transplantation. Combining single-cell and spatial profiling, organoid mapping can provide structural and molecular profiles of organoids in comparison with corresponding tissues or organs. This approach enhances the high simulation of current organoid construction and cultivation. Additionally, it contributes to further optimization of disease modeling. The establishment of organoid libraries will greatly contribute to the provision of ready-to-use disease models for drug screening. These libraries are also expected to provide immediate organoid substitutes for the treatment of malignant or advanced diseases, such as cancer. The establishment of organoid banks will greatly help to supply ready-to-use disease models for drugs screening, and is expected to provide immediate organ substitutes for treating malignant or late-stage diseases such as cancer.