Abstract
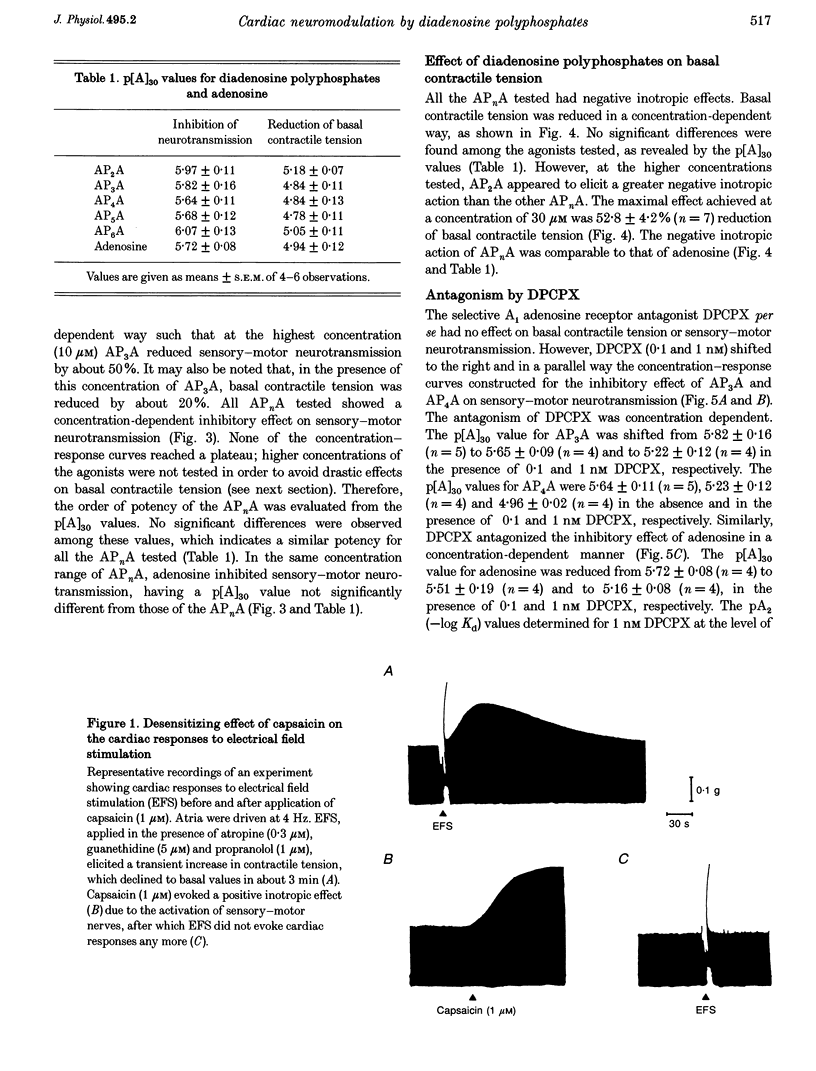
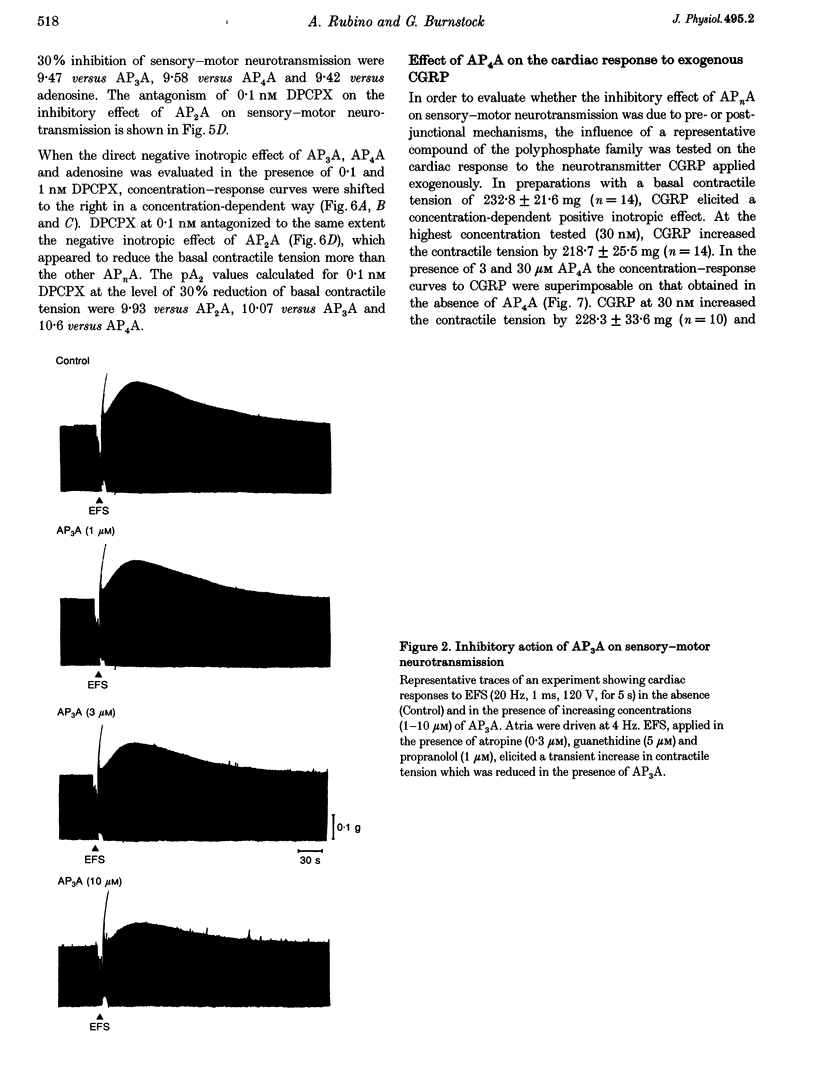
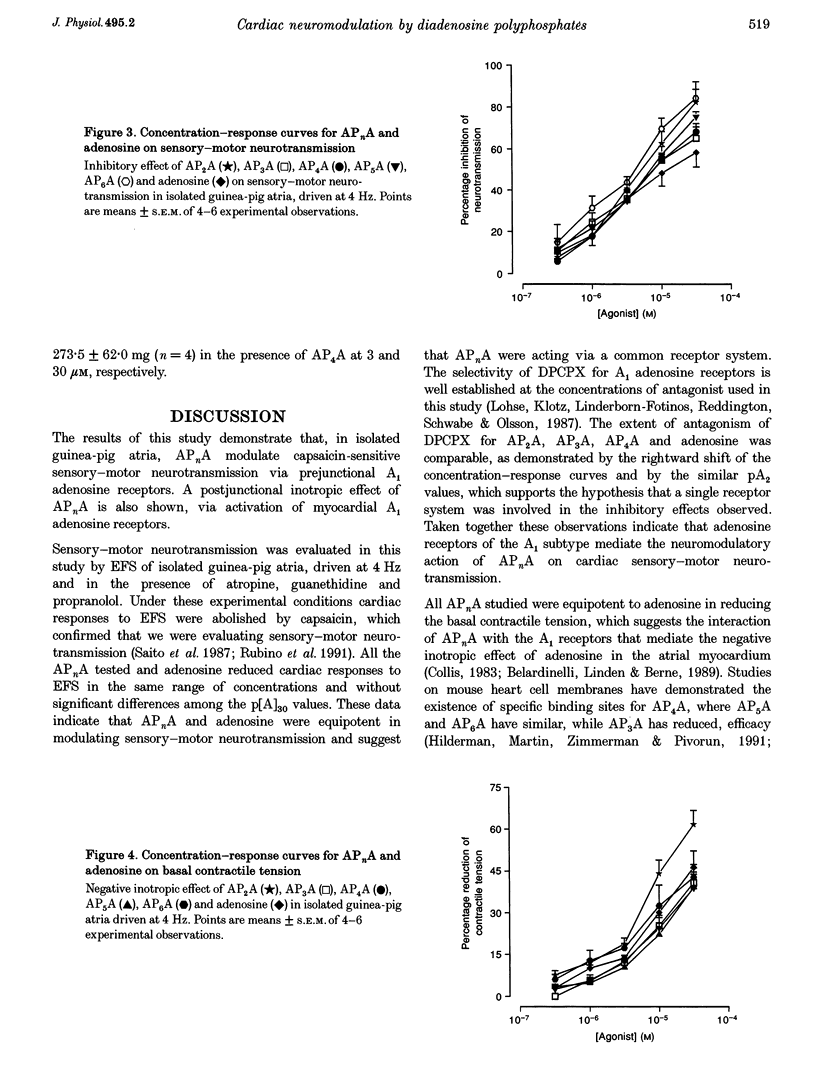
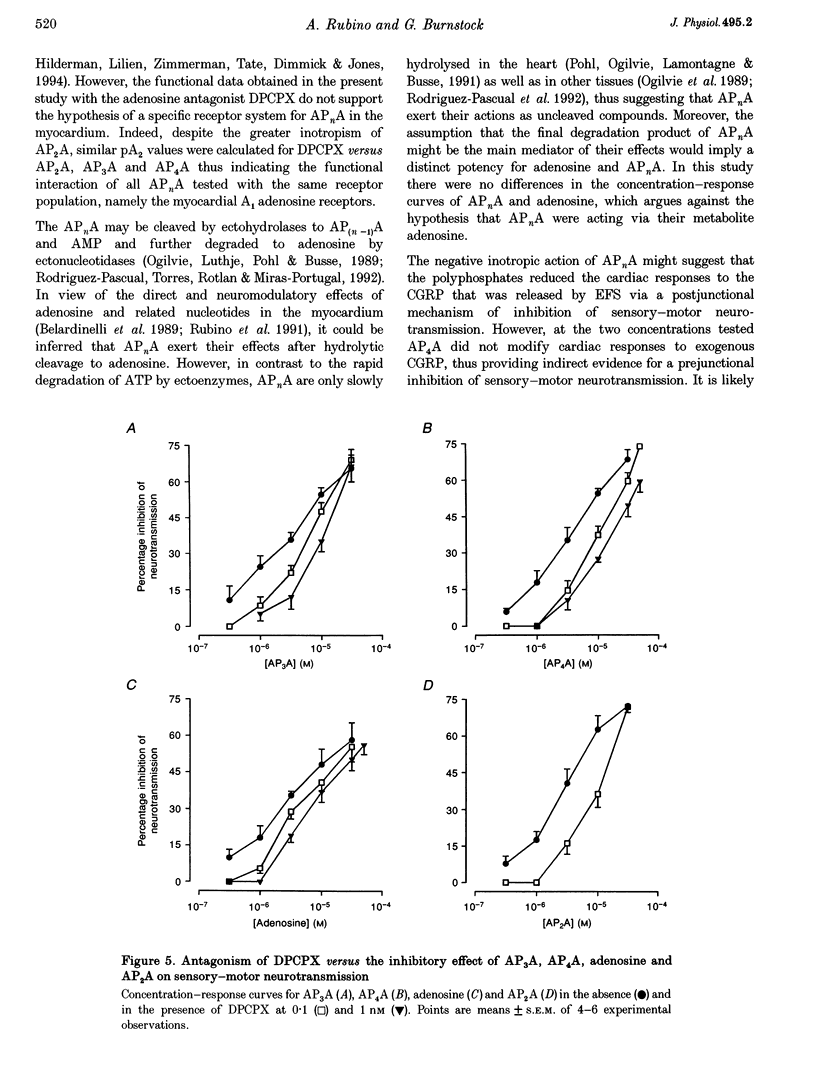
1. Isolated guinea-pig atria were used to study the neuromodulatory effect of diadenosine polyphosphates (APnA) on cardiac capsaicin-sensitive sensory-motor neurotransmission. 2. In the presence of atropine, guanethidine and propranolol, electrical field stimulation (EFS) of the atrial preparations evoked a positive inotropic response which is known to be mediated by release of calcitonin gene-related peptide (CGRP) from sensory-motor nerves. P1,P2-diadenosine pyrophosphate (AP2A), P1,P3-diadenosine triphosphate (AP3A), P1,P4-diadenosine tetraphosphate (AP4A), P1,P5-diadenosine pentaphosphate (AP5A) and P1,P6-diadenosine hexaphosphate (AP6A) inhibited in a concentration-dependent way (0.1-30 microM) cardiac responses to EFS. The inhibitory effect of APnA was mimicked by adenosine. 3. All the APnA tested had a direct negative inotropic effect, by reducing in a concentration-dependent manner the basal contractile tension. The inotropism of APnA was comparable to that of adenosine. 4. Both inhibition of cardiac responses to EFS and negative inotropism of AP2A, AP3A and AP4A were sensitive to the antagonism by the A1 adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 0.1-1 nM). The extent of antagonism of DPCPX for the APnA tested was comparable to that for adenosine. 5. Despite the direct negative inotropism, AP4A tested at the highest concentration used did not affect the cardiac responses to the neurotransmitter CGRP, applied exogenously. 6. These results have demonstrated that in isolated guinea-pig atria APnA inhibited sensory-motor neurotransmission, without affecting cardiac responses to exogenous CGRP. The effect of APnA was sensitive to antagonism by DPCPX, which suggests it operates via the activation of prejunctional A1 adenosine receptors. A postjunctional negative inotropism was also shown, mediated by myocardial A1 adenosine receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Baxi M. D., Vishwanatha J. K. Diadenosine polyphosphates: their biological and pharmacological significance. J Pharmacol Toxicol Methods. 1995 Jun;33(3):121–128. doi: 10.1016/1056-8719(94)00127-p. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Linden J., Berne R. M. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989 Jul-Aug;32(1):73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990 Mar-Apr;304:7–33. [PubMed] [Google Scholar]
- Castillo C. J., Moro M. A., Del Valle M., Sillero A., García A. G., Sillero M. A. Diadenosine tetraphosphate is co-released with ATP and catecholamines from bovine adrenal medulla. J Neurochem. 1992 Aug;59(2):723–732. doi: 10.1111/j.1471-4159.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- Castro E., Torres M., Miras-Portugal M. T., Gonzalez M. P. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990 Jun;100(2):360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G. Evidence for an A1-adenosine receptor in the guinea-pig atrium. Br J Pharmacol. 1983 Jan;78(1):207–212. doi: 10.1111/j.1476-5381.1983.tb09381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H., Klenow H. Abundant amounts of diadenosine 5',5"'-P1,P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem J. 1982 Dec 15;208(3):737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderman R. H., Lilien J. E., Zimmerman J. K., Tate D. H., Dimmick M. A., Jones G. B. Adenylated dinucleotide binding to the adenosine 5',5'''-P1,P4-tetraphosphate mouse heart receptor. Biochem Biophys Res Commun. 1994 Apr 29;200(2):749–755. doi: 10.1006/bbrc.1994.1514. [DOI] [PubMed] [Google Scholar]
- Hilderman R. H., Martin M., Zimmerman J. K., Pivorun E. B. Identification of a unique membrane receptor for adenosine 5',5"'-P1,P4-tetraphosphate. J Biol Chem. 1991 Apr 15;266(11):6915–6918. [PubMed] [Google Scholar]
- Klishin A., Lozovaya N., Pintor J., Miras-Portugal M. T., Krishtal O. Possible functional role of diadenosine polyphosphates: negative feedback for excitation in hippocampus. Neuroscience. 1994 Jan;58(2):235–236. doi: 10.1016/0306-4522(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Ogilvie A. The presence of diadenosine 5',5'''-P1,P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun. 1983 Aug 30;115(1):253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- Mair J., Lechleitner P., Längle T., Wiedermann C., Dienstl F., Saria A. Plasma CGRP in acute myocardial infarction. Lancet. 1990 Jan 20;335(8682):168–168. doi: 10.1016/0140-6736(90)90040-c. [DOI] [PubMed] [Google Scholar]
- Ogilvie A., Lüthje J., Pohl U., Busse R. Identification and partial characterization of an adenosine(5')tetraphospho(5')adenosine hydrolase on intact bovine aortic endothelial cells. Biochem J. 1989 Apr 1;259(1):97–103. doi: 10.1042/bj2590097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Díaz-Rey M. A., Torres M., Miras-Portugal M. T. Presence of diadenosine polyphosphates--Ap4A and Ap5A--in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci Lett. 1992 Mar 2;136(2):141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- Pintor J., Miras-Portugal M. T. A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br J Pharmacol. 1995 Jul;115(6):895–902. doi: 10.1111/j.1476-5381.1995.tb15894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Porras A., Mora F., Miras-Portugal M. T. Amphetamine-induced release of diadenosine polyphosphates--Ap4A and Ap5A--from caudate putamen of conscious rat. Neurosci Lett. 1993 Feb 5;150(1):13–16. doi: 10.1016/0304-3940(93)90096-4. [DOI] [PubMed] [Google Scholar]
- Pohl U., Ogilvie A., Lamontagne D., Busse R. Potent effects of AP3A and AP4A on coronary resistance and autacoid release of intact rabbit hearts. Am J Physiol. 1991 May;260(5 Pt 2):H1692–H1697. doi: 10.1152/ajpheart.1991.260.5.H1692. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Presence of diadenosine 5',5''' -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive "pleiotypic activator". Proc Natl Acad Sci U S A. 1976 Nov;73(11):3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez del Castillo A., Torres M., Delicado E. G., Miras-Portugal M. T. Subcellular distribution studies of diadenosine polyphosphates--Ap4A and Ap5A--in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988 Dec;51(6):1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pascual F., Torres M., Rotllán P., Miras-Portugal M. T. Extracellular hydrolysis of diadenosine polyphosphates, ApnA, by bovine chromaffin cells in culture. Arch Biochem Biophys. 1992 Aug 15;297(1):176–183. doi: 10.1016/0003-9861(92)90657-i. [DOI] [PubMed] [Google Scholar]
- Rubino A., Amerini S., Mantelli L., Ledda F. Adenosine receptors involved in the inhibitory control of non-adrenergic non-cholinergic neurotransmission in guinea-pig atria belong to the A1 subtype. Naunyn Schmiedebergs Arch Pharmacol. 1991 Oct;344(4):464–470. doi: 10.1007/BF00172587. [DOI] [PubMed] [Google Scholar]
- Rubino A., Burnstock G. Capsaicin-sensitive sensory-motor neurotransmission in the peripheral control of cardiovascular function. Cardiovasc Res. 1996 Apr;31(4):467–479. [PubMed] [Google Scholar]
- Rubino A. Non-adrenergic non-cholinergic (NANC) neural control of the atrial myocardium. Gen Pharmacol. 1993 May;24(3):539–545. doi: 10.1016/0306-3623(93)90210-o. [DOI] [PubMed] [Google Scholar]
- Saito A., Ishikawa T., Kimura S., Goto K. Role of calcitonin gene-related peptide as cardiotonic neurotransmitter in guinea pig left atria. J Pharmacol Exp Ther. 1987 Nov;243(2):731–736. [PubMed] [Google Scholar]
- Stone T. W., Perkins M. N. Adenine dinucleotide effects on rat cortical neurones. Brain Res. 1981 Dec 14;229(1):241–245. doi: 10.1016/0006-8993(81)90764-2. [DOI] [PubMed] [Google Scholar]


