FIG. 3.
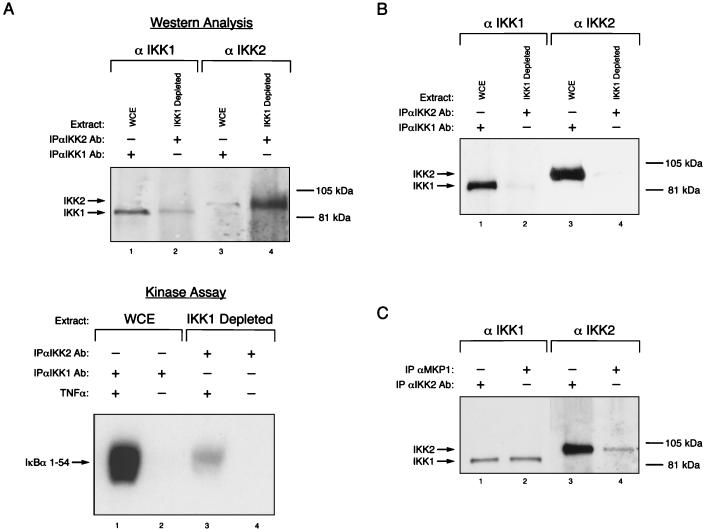
The IKK signalsome displays cell-type-specific heterogeneity in subunit composition. (A) IKK1 depletion studies further substantiate the existence of distinct IKK-containing pools. (Top) Whole-cell extract (WCE) from TNF-α-stimulated HeLa cells was depleted of IKK1-containing complexes by immunoprecipitation with an excess of anti-IKK1-specific antibodies (Ab) (lanes 1 and 3). The IKK1-depleted supernatant (IKK1 Depleted) was subsequently immunoprecipitated with anti-IKK2 antibody (lanes 2 and 4). Immunoprecipitates were resolved by SDS-PAGE and subjected to Western blot analysis with anti-IKK1-specific and anti-IKK2-specific antibodies, as indicated. (Bottom) Immunoprecipitations were performed with WCEs of HeLa cells that were stimulated with TNF-α (lanes 1 and 3) or not stimulated (lanes 2 and 4) as described for the top panel. The immunoprecipitates were then analyzed for IκBα kinase activity with GST IκBα 1–54 as the substrate. (B) SLB cells do not contain detectable levels of the IKK2 homodimeric complex. Whole-cell extracts were prepared from unstimulated SLB cells and analyzed for the presence of IKK1-IKK2 and IKK2-only complexes exactly as described for panel A above. (C) The IKK signalsome component recognized by the anti-MKP antibody is specific for the IKK1-IKK2 heterodimeric complex. Immunoprecipitations were performed from whole-cell extracts prepared from TNF-α-induced HeLa cells by using either anti-MKP1 (lanes 2 and 4) or anti-IKK2-specific (lanes 1 and 3) antibodies. The immunoprecipitates were resolved by SDS-PAGE and subjected to Western blot analysis with either IKK1-specific or IKK2-specific antibodies, as indicated.