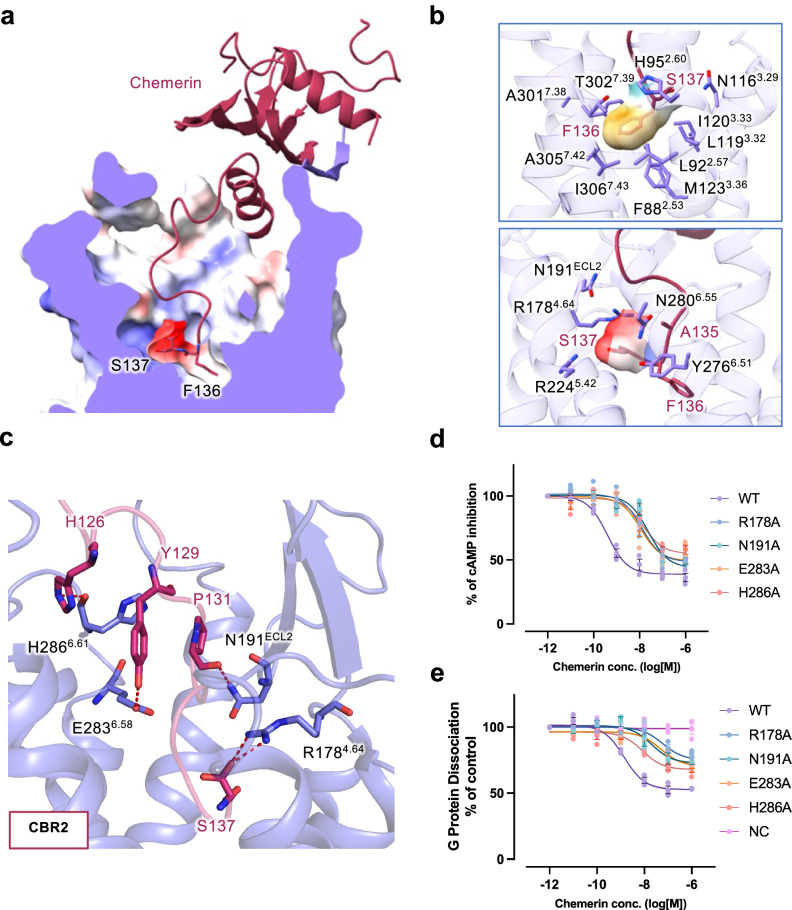
Fig. 3. Molecular interactions in the transmembrane pocket of CMKLR1.
a Sliced view of the transmembrane binding pocket of CMKLR1. The C-terminal two residues of chemerin (S137 and F136) are shown in sticks. Electrostatic potential surface is shown in the binding pocket. b Hydrophobic and negatively-charged pocket for the C-terminal two amino acids of chemerin. In the upper panel, F136 is surrounded by a hydrophobic binding pocket. In the lower panel, S137 fits in a negatively-charged binding pocket. c Polar interactions at the chemerin binding region 2 (CBR2) in the transmembrane binding pocket of CMKLR1. Hydrogen bonds are shown in red dashes. d cAMP inhibition assay in cells expressing wild-type and mutant CMKLR1. e NanoBiT-based G protein dissociation assay in cells expressing wild-type and mutant CMKLR1. Data are shown as mean ± SEM from n = 3 independent experiments.