Abstract
Endovascular treatment (EVT) is the first-line treatment for petrous ridge dural arteriovenous fistulas (DAVFs). However, EVT is associated with complications. Among these complications, delayed venous hemorrhage is fatal. Here, we report such a case in a 59-year-old male with a 1-month history of dizziness. Previously, the patient was healthy. Physical examination showed no abnormalities. Computed tomography (CT) angiography revealed a petrous ridge DAVF that was draining via the superior petrosal venous complex and superior petrosal sinus, venous drainage involving the venous system of the brainstem, and 2 aneurysmal dilatations on the brainstem vein. EVT was performed via the ascending pharyngeal artery to cast Onyx-18, and the DAVF was obliterated. During EVT, the venous system of the brainstem was impaired by the occlusion of the aneurysmal dilatation. Postoperatively, the patient awoke. Twenty hours after EVT, he experienced left hemiplegia, and CT revealed no hemorrhage. However, thirty hours after EVT, the patient fell into a deep coma, and CT revealed hemorrhage of the brainstem and cerebellum into the ventricle system. Delayed venous hemorrhage was considered. After receiving conservative treatment for 10 hours, the patient died. This case demonstrates that excessive occlusion of the draining vein of a DAVF may result in fatal delayed venous hemorrhage. To decrease this risk, staged embolization may be useful after occluding the high-risk draining vein or reducing the DAVF blood flow. In conclusion, during EVT for petrous ridge DAVFs, care should be taken not to impair the venous system of the brainstem, to prevent venous hemorrhage.
Keywords: Petrous ridge, Dural arteriovenous fistula, Endovascular treatment, Delayed hemorrhage
Introduction
Dural arteriovenous fistulas (DAVFs) are arteriovenous shunts located within the dural leaflet. Petrous ridge DAVFs are rare, accounting for only 0.5%-4.5% of all intracranial DAVFs [1]. Petrous ridge DAVFs may rupture and present with intracranial hemorrhage due to a high Cognard classification following deep venous drainage. Therefore, treatment is often necessary. Currently, endovascular treatment (EVT) is the first-line therapy for intracranial DAVFs. However, EVT is difficult and dangerous to perform in patients with petrous ridge DAVFs because of the complex angioarchitectures involved [2]. EVT for petrous ridge DAVFs may be associated with complications. Among these complications, delayed venous hemorrhage is a rare and fatal event [3].
Case presentation
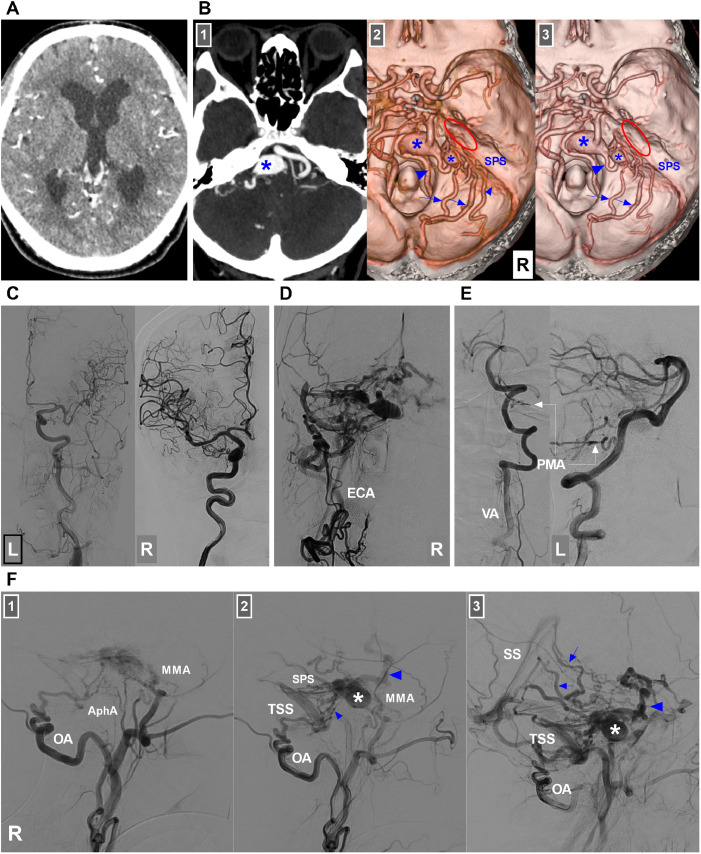
A 59-year-old male of Han Chinese nationality presented with a 1-month history of dizziness. Previously, he was healthy and had no history of hypertension or diabetes mellitus. Physical examination revealed no abnormal findings. The patient had grade V muscle strength in his upper and lower limbs. Computed tomography (CT) revealed mild ventriculomegaly (Fig. 1A). CT angiography (CTA) revealed a petrous ridge DAVF draining into the superior petrosal sinus (SPS), anterior and lateral brainstem veins and cerebellar cortical veins via the superior petrosal venous complex (SPVC), as well as 2 aneurysmal dilatations on the anterior brainstem vein (Fig. 1B). The DAVF was classified as Cognard type IV.
Fig. 1.
CT, CTA and diagnostic DSA images. (A) Enhanced CT image showing mild ventriculomegaly. (B) CTA images: Number 1 panel: Maximum intensity projection showing a mass effect (asterisk) in front of the brainstem. Panels 2 and 3: CTA image showing a petrous ridge DAVF (ellipses) with venous drainage into the SPS and a superior petrosal venous complex into the anterior pontomesencephalic vein (large arrowheads) with 2 aneurysmal dilatations (asterisks), lateral pontine veins (arrows) and cerebellar cortical veins (small arrowheads). (C) DSA images of the left carotid artery (left panel) and right internal carotid artery (right panel) showing no feeders to the DAVF. (D) DSA image of the right ECA confirming the DAVF. (E) DSA image of the left VA showing the PMA (arrows) feeding the DAVF. (F) DAVF angioarchitecture: Early arterial (number 1 panel), arterial (number 2 panel) and venous (number 3 panel) phases of DSA images of the right ECA showing that the DAVF was fed by the MMA, AphA and OA; its venous drainage had multiple paths, including the SPS, anterior brainstem vein (large arrowheads), cerebellar cortical veins (small arrowheads) into the TSS, and lateral brainstem veins (arrows) into the SS. Aneurysmal dilatation can be observed in the draining vein (asterisks). Abbreviations: AphA, ascending pharyngeal artery; CT, computed tomography; CTA, CT angiography; DAVF, dural arteriovenous fistula, DSA, digital subtraction angiography; ECA, external carotid artery; L, left; MMA, middle meningeal artery; OA, occipital artery; PMA, posterior meningeal artery; R, right; SPS, superior petrosal sinus; SS, straight sinus; TSS, transverse-sigmoid sinus; VA, vertebral artery.
Consequently, EVT was planned. Under general anesthesia, digital subtraction angiography (DSA) confirmed a petrous ridge DAVF, and the feeding arteries arose from the right middle meningeal artery (MMA), right ascending pharyngeal artery (AphA), right occipital artery (OA) and left posterior meningeal artery (Fig. 1C-F).
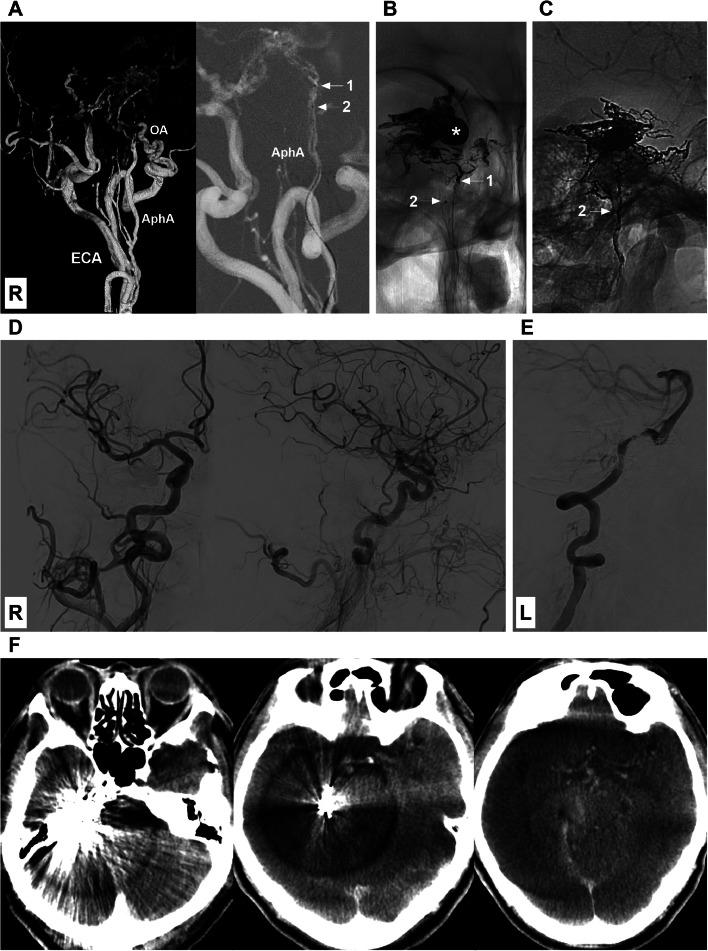
EVT was subsequently performed. After an 8F guiding catheter was placed at the AphA orifice, 2 Marathon microcatheters (Medtronic, Minneapolis, MI, USA) reached the distal AphA under the guidance of a Synchro 10 micro-guidewire (Stryker Neurovascular, Fremont, CA, USA) (Fig. 2A). Then, 4 ml of Onyx-18 (Medtronic, Irvine, CA, USA) was injected via 2 Marathon microcatheters (Fig. 2B-C), and the DAVF was obliterated (Fig. 2D-E). During the EVT, the aneurysmal dilatation of the anterior brainstem vein was occluded by Onyx (Fig. 2B), which impaired the venous system of the brainstem. Therefore, Xper-CT was performed immediately after the operation, and no signs of hemorrhage were detected (Fig. 2F).
Fig. 2.
Endovascular treatment of the DAVF. (A) Left panel: Three-dimensional digital subtraction angiography (DSA) image showing that the AphA may be a potential route for embolization of the DAVF because catheterization is easy to access the fistula. Right panel: Roadmap image showing successful catheterization of AphA with 2 Marathon microcatheters (arrows with numbers 1 and 2). (B) X-ray image showing that the DAVF was partially embolized by casting Onyx via the microcatheter labeled by an arrow with the numeral 1, and the venous aneurysmal dilatation was filled with Onyx (asterisk). (C) X-ray image showing the Onyx casing in the DAVF after complete embolization via the microcatheter labeled by an arrow with the numeral 2. (D-E) DSA images of the right carotid artery and left vertebral artery showing that the DAVF was obliterated. (F) Xper-CT images showing no hemorrhage after embolization. Abbreviations: AphA, ascending pharyngeal artery; DSA, digital subtraction angiography; ECA, external carotid artery; L, left; OA: occipital artery; R, right.
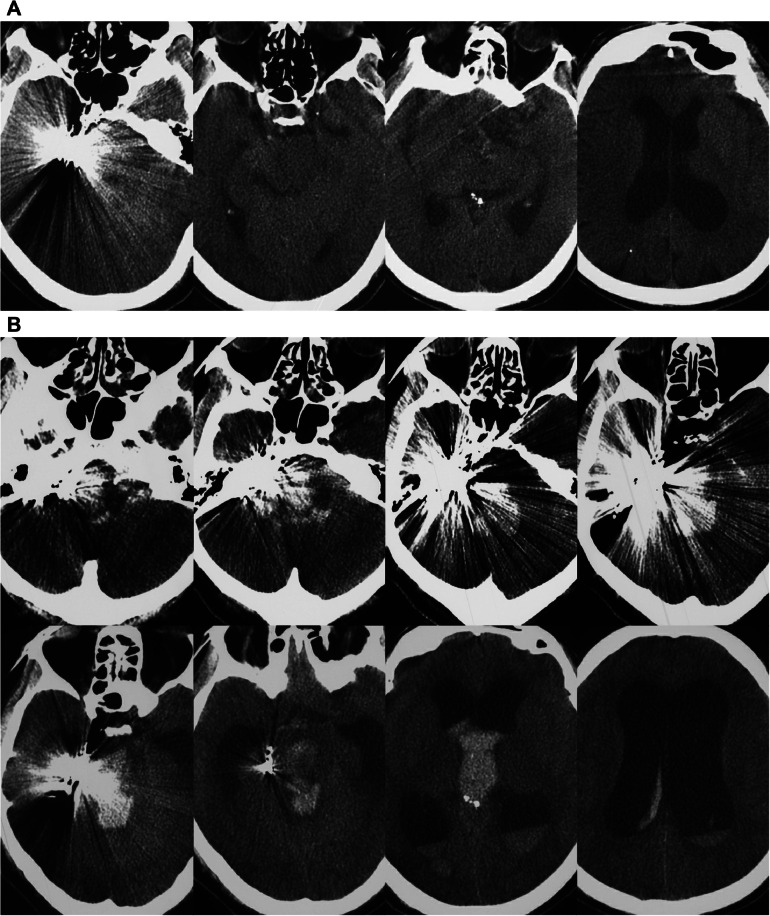
One hour after surgery, the patient awoke. No new neurologic deficits were detected, and the patient exhibited grade V muscle strength in his arms and legs. However, 20 hours after EVT, the patient began experiencing left hemiplegia, as indicated by grade II muscle strength in his arm and grade IV muscle strength in his leg. A CT examination was performed, and no hemorrhage was detected (Fig. 3A). Therefore, brain ischemia was suspected, and magnetic resonance imaging was planned. A nasogastric feeding tube was placed to maintain an adequate fluid volume for good circulation. However, the patient's condition did not improve. Thirty hours after EVT, the patient fell into a deep coma, and CT was performed, this time revealing hemorrhage of the brainstem and cerebellum into the ventricle system and hydrocephalus (Fig. 3B). A diagnosis of venous hemorrhage was considered; however, his family refused external ventricular drainage and other invasive treatment. After receiving conservative therapy for 10 hours, the patient died.
Fig. 3.
Postoperative CT examination. (A) Twenty hours after treatment, CT revealed no hemorrhage. (B) Thirty hours after treatment, CT imaging showed hemorrhage of the brainstem and cerebellum into the ventricle system and hydrocephalus. Abbreviations: CT, computed tomography.
Discussion
Petrous ridge DAVFs are also known as SPS DAVFs. These lesions may develop from the opening of existing microshunts within the dura or as a result of angioneogenesis, which tends to lead to the development of new shunts. Petrosal ridge DAVFs are located in the dura at the connection between the SPVC and SPS. These DAVFs are associated with the SPS and drain into the SPVC; moreover, they can be divided into high-flow and low-flow types. High-flow fistulas can develop as a result of arterial overflow, whereas low-flow fistulas develop due to high venous pressure [4]. Owing to the anatomical characteristics of the petrous ridge region, the main feeding artery of petrous ridge DAVFs may arise from the MMA, AphA, and OA, and the main draining vein may flow into the SPS, SPVC and its tributaries [2].
Petrous ridge DAVFs are classified into high-Cognard types due to SPVC involvement (Figs. 1 B, D and F). SPVC involvement results in both cortical and deep venous drainage. Hemorrhage is strongly correlated with these types of venous drainage [5]. Therefore, further treatment is recommended. Currently, EVT is considered the first-line treatment for DAVFs. In EVT for DAVFs, all arteriovenous connections between the arterial and venous sides should be closed, and the proximal draining vein should be better sealed to cure the abnormal connection. For DAVFs, transarterial embolization (TAE) or transvenous embolization (TVE) can resolve the abnormal connection, depending on the accessible approach (Figs. 4 and 5).
Fig. 4.
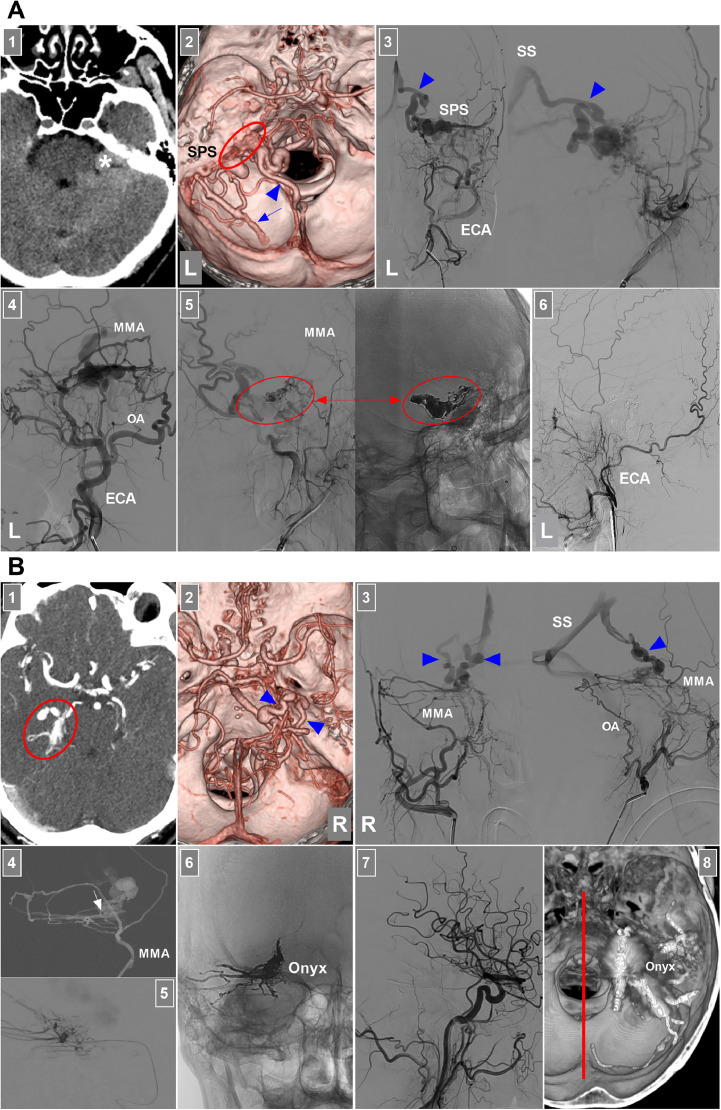
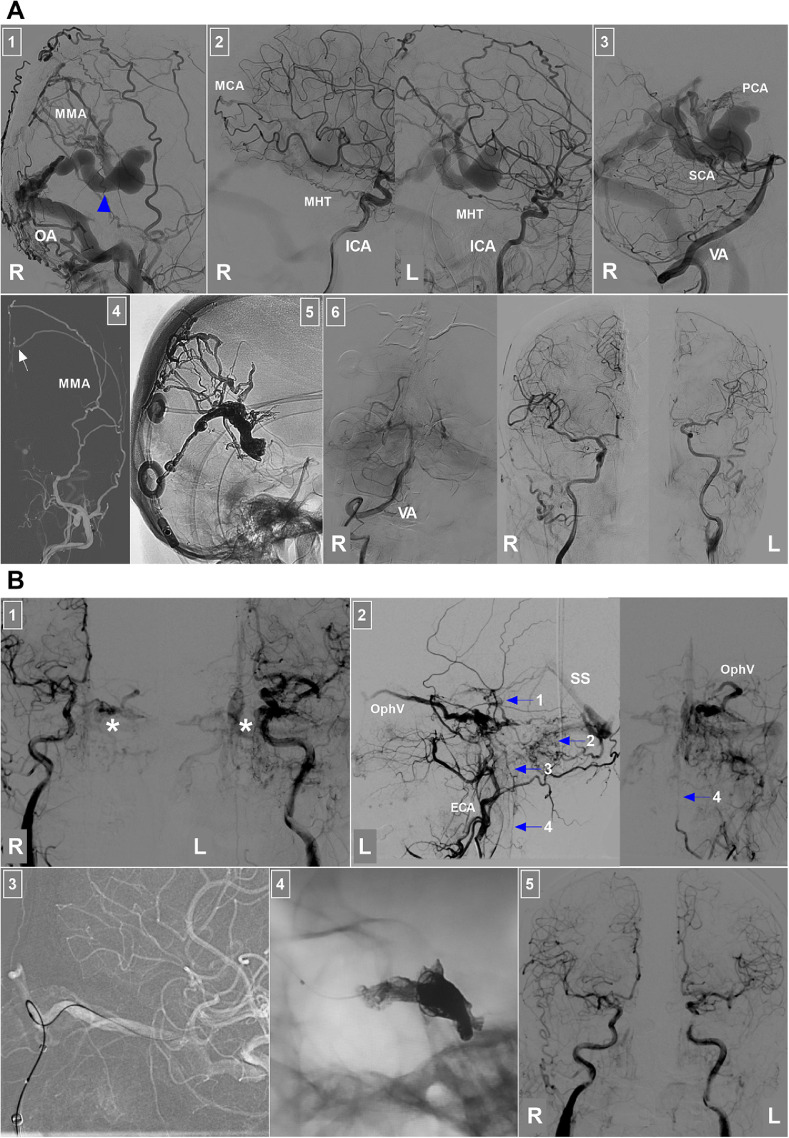
EVT of DAVFs around the brainstem. (A) EVT of a petrous ridge DAVF: Panel 1: CT image showing a lesion (asterisk) behind the petrous ridge; Panel 2: CTA image showing that a left petrous ridge DAVF (ellipse) drained into the superior petrosal venous complex and SPS, lateral brainstem vein (arrowhead) and cerebellar cortical vein (arrow); Panel 3: Anterior posterior (left) and lateral (right) views of the DSA of the left ECA confirming the petrous ridge DAVF; the lateral brainstem vein (arrowhead) was the main drainer into the SS; Panel 4: DSA of the left ECA showing that the MMA and OA supplied the DAVF; MMA was a good route through which to embolize the DAVF; Panel 5: DSA (left) and X-ray (right) images showing the delivery of Onyx (ellipses and arrows) into the DAVF via the MMA not involving the draining vein; Panel 6: Six-month follow-up DSA of the left ECA showing no residual DAVF. (B) EVT of the tentorial incisural DAVF: Panel 1: Enhanced CT image showing a lesion of the right tentorial incisura (ellipse); Panel 2: CTA image showing 2 main deep draining veins (arrowheads) into the SS, where the veins were dilated and tortuous; Panel 3: Anterior posterior (left) and lateral (right) views of the DSA of the right ECA confirming that the tentorial incisural DAVF had the MMA and OA as the feeder and the deep veins (arrowheads) as the drainer into the SS; Panel 4: Roadmap image showing that the microcatheter (arrow) in the MMA accessed the fistula; Panel 5: Microcatheter angiography showing the fistula; Panel 6: X-ray image showing the Onyx casing in the DAVF not involving too much of the draining vein; Panel 7: DSA showing that the DAVF was obliterated; Panel 8: Xper-reconstructive CT showing the Onyx location, where the red line indicates the midline. Abbreviations: CT, computed tomography; CTA, CT angiography; DAVF, dural arteriovenous fistula; DSA, digital subtraction angiography; ECA, external carotid artery; EVT, endovascular treatment; L, left; MMA, middle meningeal artery; OA, occipital artery, R, right; SPS, superior petrosal sinus; SS, straight sinus.
Fig. 5.
EVT of DAVFs around the brainstem. (A) EVT of a tentorial Galenic DAVF: Panel 1: DSA of the right ECA showing that the DAVF was fed by the MMA and OA; the arrowhead indicates the vein of Galen as the drainer; Panel 2: DSA images of bilateral ICAs showing that the DAVF was fed by the right MCA termination and bilateral MHTs; Panel 3: DSA of the right VA showing the meningeal branches of the PCA and the SCA supplied the DAVF; Panel 4: Roadmap image showing the microcatheter (arrow) in the parietal branch of the MMA accessing the DAVF; Panel 5: X-ray image showing Onyx casting in the DAVF; Panel 6: DSA images of the right VA and bilateral carotid arteries showing that the DAVF was obliterated. (B) EVT of a cavernous DAVF: Panel 1: DSA images of bilateral carotid arteries showing a cavernous DAVF (asterisks); Panel 2: DSA images of the left ECA showing the draining veins of the DAVF, including the OphV, basal vein into the SS (Arrow 1), cerebellar vein (Arrow 2), brainstem vein (Arrow 3) and spinal vein (Arrow 4); Panel 3: Roadmap image showing that the superior OphV was catheterized successfully; Panel 4: X-ray image showing that Onyx casting and coiling packed the cavernous sinus; Panel 5: DSA images of the bilateral carotid arteries showing that the DAVF was obliterated. Abbreviations: DAVF, dural arteriovenous fistula; DSA, digital subtraction angiography; ECA, external carotid artery; EVT, endovascular treatment; ICA, internal carotid artery; L, left; MCA, middle cerebral artery; MMA, middle meningeal artery; MHT, meningohypophyseal trunk; OA, occipital artery; OphV, ophthalmic vein; PCA, posterior cerebral artery; R, right; SCA, superior cerebellar artery; SS, straight sinus; VA, vertebral artery.
TAE can be performed for petrous ridge DAVFs with large and straight arterial feeders. For most intracranial DAVFs, TAE via the MMA is the best strategy [6]. There is no exception for DAVFs in the petroclival region. For example, in the case shown in Fig. 4A and in Alleyne et al.’s study of SPS DAVFs, TAE via the MMA had a curative effect [7]. However, in our patient with a petrous ridge DAVF (Fig. 1, Fig. 2), the MMA was tortuous and hypoplastic. Therefore, the AphA was selected for TAE (Figs. 2A-C). In select cases, TAE via the AphA may be a suitable treatment strategy for DAVFs. In Gross et al.’s report, of 267 treated DAVFs, 68 (25%) had an AphA supply. Embolization was performed via the AphA in 8 (12%) of these 68 DAVFs, and 7 were ultimately occluded. No post-EVT cranial neuropathies or radiographic evidence of nontarget embolization was found [8].
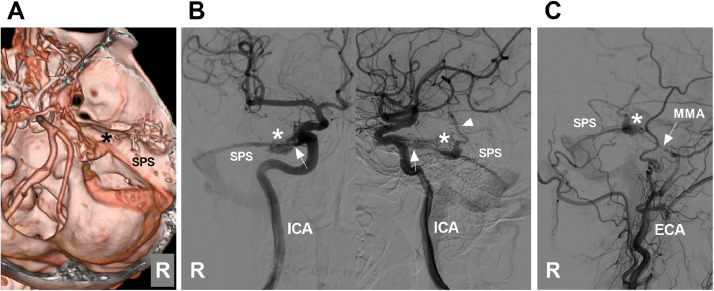
For petrous ridge DAVFs, TVE should also be reserved for cases in which the fistula cannot be reached via the feeding artery. For petrous ridge DAVFs with SPS drainage, the SPS can be a suitable delivery pathway for embolic agents (Fig. 6) [9]. However, stenosis of the downstream SPS or significant stenosis proximal to its junction with the sigmoid sinus can prevent access to the fistula site [10].
Fig. 6.
A petrous ridge DAVF that is appropriate for TVE. (A) CTA image showing a petrous ridge DAVF (asterisk). (B) Anterior posterior (left panel) and lateral (right panel) views of DSA of the right ICA showing that the DAVF (asterisks) was supplied by the MHT (arrows) and drained into the SPS and a deep vein (arrowhead). (C) DSA of the right ECA showing that the slim MMA (arrow) also supplied the DAVF (asterisk). Abbreviations: CTA, computed tomography angiography; DAVF, dural arteriovenous fistula; DSA, digital subtraction angiography; ICA, internal carotid artery; MHT, meningohypophyseal trunk; MMA, middle meningeal artery; R, right; SPS, superior petrosal sinus; TVE, transvenous embolization.
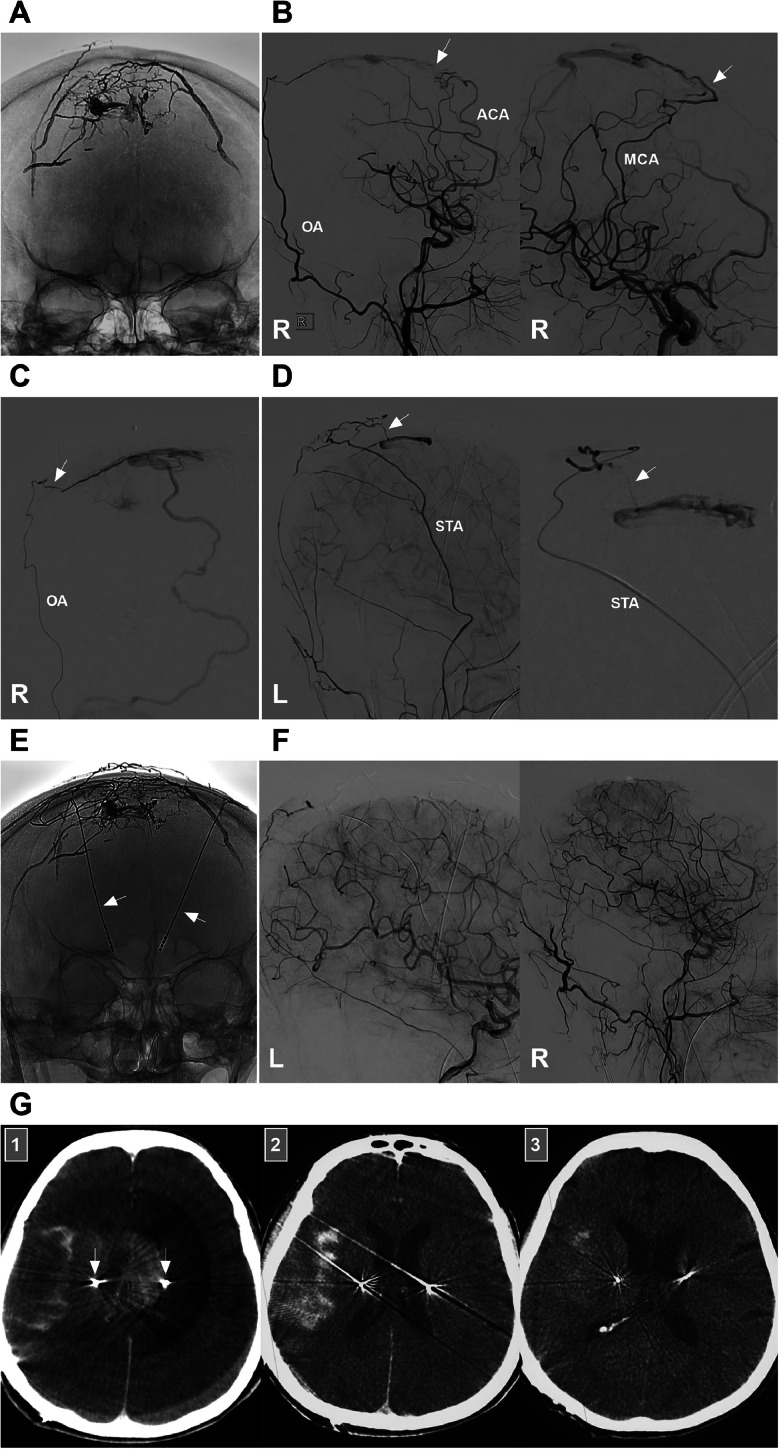
EVT for petrous ridge DAVFs can lead to various complications. Among all the complications, delayed venous hemorrhage is the most serious. In this case report, the patient experienced such a complication and died (Fig. 3). Venous hemorrhage may be associated with the rupture of a glomus-like structure around the draining vein, which is often supplied by a pial artery (Fig. 7). In addition, excessive occlusion of the ostium of drainage veins may result in venous hypertension, which increases the risk of venous hemorrhage [3]. In our patient, the DAVF had no pial arterial feeder, and the delayed venous hemorrhage was likely caused by impairment of the venous system of the brainstem.
Fig. 7.
SAH complication of EVT for a superior sagittal sinus DAVF. (A) X-ray image showing previous Onyx casting to treat superior sagittal sinus DAVF; (B) DSA images of the right carotid artery showing that the residual DAVF was supplied by the right ACA (arrow) (left panel) and MCA (arrow) (right panel); (C) DSA of the right OA showing that the residual DAVF was supplied by the OA via the transosseous branch (arrow); (D) DSA images of the left ECA (left panel) and STA (right panel) showing that the residual DAVF was supplied by the STA via the transosseous branch (arrow); (E) X-ray image showing the Onyx casting after the residual DAVF was embolized via the ACA, MCA, OA and STA; the arrows indicate the microelectrodes used in operations for Parkinson's disease; (F) DSA images of the bilateral carotid arteries showing that the DAVF was obliterated; (G) Post-EVT immediate Xper-CT (Panel 1) and CT of 6 hours after EVT (Panel 2) showing SAH in the right sylvian fissure; Panel 3: CT showing SAH absorption after 48 hours of EVT; arrows indicate the microelectrodes. Abbreviations: ACA, anterior cerebral artery; DAVF, dural arteriovenous fistula; DSA, digital subtraction angiography; ECA, external carotid artery; EVT, endovascular treatment; L, left; MCA, middle cerebral artery; OA, occipital artery; R, right; SAH, subarachnoid hemorrhage; STA, superior temporal artery.
For DAVFs, not all occlusions of the proximal draining vein can be associated with venous hemorrhage. When treating DAVFs around the brainstem, if the draining vein is isolated and does not communicate with veins with normal draining function, even if the proximal draining vein is occluded by an embolic agent, EVT is safe (Fig. 4). In addition, if only the cavernous sinus is occluded during the treatment of DAVFs around the brainstem, EVT is considered safe (Fig. 5). The SPVC is a complex venous structure that drains into the SPS. It has 1-5 possible major tributaries: the vein of the cerebellopontine fissure, the vein of the middle cerebellar peduncle, the pontotrigeminal vein, the transverse pontine vein, and the anterior lateral marginal vein [11,12]. When the SPVC is involved in a petrous ridge DAVF, if brainstem tributaries of the SPVC are involved in venous drainage, they must be protected, and the liquid embolic agent is not allowed to fully occlude and impair the brainstem vein, which can cause severe venous hemorrhage, such as in the present patient.
To decrease such delayed hemorrhagic complications, staged embolization may be useful after occlusion of the high-risk draining vein or reduction of the DAVF blood flow. Delayed hemorrhagic complications are often serious because of the nature of venous hypertension. If the hemorrhage is located in a noneloquent region and can be evacuated after emergency craniotomy, an acceptable outcome can be achieved. However, in our case, the hemorrhage involved the brainstem and deep cerebellum, and the outcome was not good. After conservative treatment, the patient died.
Conclusion
Although the exact cause of delayed venous hemorrhage in petrous ridge DAVFs is unclear, impaired draining function of the SPVC may be a cause. When brainstem tributaries of the SPVC are involved in venous drainage, the venous system of the brainstem should not be impaired, to prevent delayed venous hemorrhage.
Patient consent
Written and informed consents were obtained from the patients for publication of this case report.
Ethics approval and consent to participate
Ethics approval was not needed in our institution, as the manuscript was a case report. Informed signed consents to participate were obtained from the patients.
Footnotes
Acknowledgments: No funding was received.
Competing Interests: The authors declare no competing interests.
References
- 1.Nishihori M, Izumi T, Tsukada T, Kato Y, Uda K, Yokoyama K, et al. Contrast-enhanced magnetic resonance imaging suggested a possibility of transvenous embolization in the superior petrosal sinus dural arteriovenous fistula: a case report. J Neuroendovasc Ther. 2022;16(3):163–169. doi: 10.5797/jnet.cr.2021-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou K, Lv X, Qu L, Guo Y, Xu K, Yu J. Endovascular treatment for dural arteriovenous fistulas in the petroclival region. Int J Med Sci. 2020;17(18):3020–3030. doi: 10.7150/ijms.47365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou K, Yu J. In: Intracranial and spinal dural arteriovenous fistulas. Lv X, editor. Springer Nature Singapore; Singapore: 2022. Hemorrhagic complications after endovascular treatment for intracranial dural arteriovenous fistulas; pp. 285–301. editor. [Google Scholar]
- 4.Pichierri A, Delfini R. Incisural and superior petrous dural arteriovenous fistulas: a contemporary perspective. World Neurosurg. 2012;77(3-4):472–474. doi: 10.1016/j.wneu.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Gomez J, Amin AG, Gregg L, Gailloud P. Classification schemes of cranial dural arteriovenous fistulas. Neurosurg Clin N Am. 2012;23(1):55–62. doi: 10.1016/j.nec.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Su H, Xu K, Wang Y, Yu J. Is the middle meningeal artery the optimal path for dural arteriovenous fistula embolization? Front Neurol. 2021;12 doi: 10.3389/fneur.2021.675355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alleyne CH, Jr., Numaguchi Z, Wang HZ. Transarterial embolisation of dural arteriovenous fistula involving an isolated segment of the superior petrosal sinus. A case report. Interv Neuroradiol. 2000;6(4):337–341. doi: 10.1177/159101990000600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross BA, Albuquerque FC, Moon K, McDougall CG. The road less traveled: transarterial embolization of dural arteriovenous fistulas via the ascending pharyngeal artery. J Neurointerv Surg. 2017;9(1):97–101. doi: 10.1136/neurintsurg-2016-012488. [DOI] [PubMed] [Google Scholar]
- 9.Hirata K, Tsuda K, Fujita K, Ishikawa E, Matsumaru Y. Transvenous embolization for isolated superior petrosal sinus dural arteriovenous fistula. NMC Case Rep J. 2024;11:7–11. doi: 10.2176/jns-nmc.2023-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng PP, Halbach VV, Quinn R, Balousek P, Caragine LP, Dowd CF, et al. Endovascular treatment for dural arteriovenous fistulae of the superior petrosal sinus. Neurosurgery. 2003;53(1):25–32. doi: 10.1227/01.neu.0000068790.37318.24. [DOI] [PubMed] [Google Scholar]
- 11.Basamh M, Sinning N, Kehler U. Individual variations of the superior petrosal vein complex and their microsurgical relevance in 50 cases of trigeminal microvascular decompression. Acta Neurochir (Wien) 2020;162(1):197–209. doi: 10.1007/s00701-019-04109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanriover N, Abe H, Rhoton AL, Jr., Kawashima M, Sanus GZ, Akar Z. Microsurgical anatomy of the superior petrosal venous complex: new classifications and implications for subtemporal transtentorial and retrosigmoid suprameatal approaches. J Neurosurg. 2007;106(6):1041–1050. doi: 10.3171/jns.2007.106.6.1041. [DOI] [PubMed] [Google Scholar]