Abstract
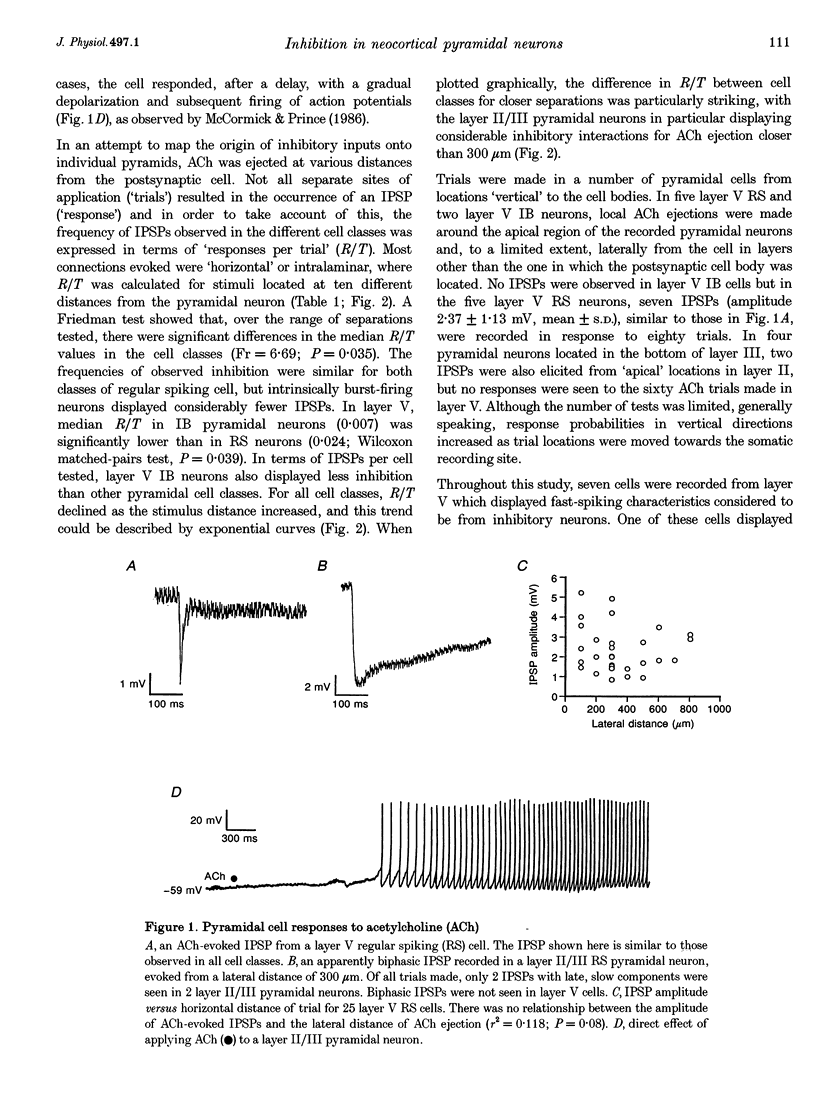
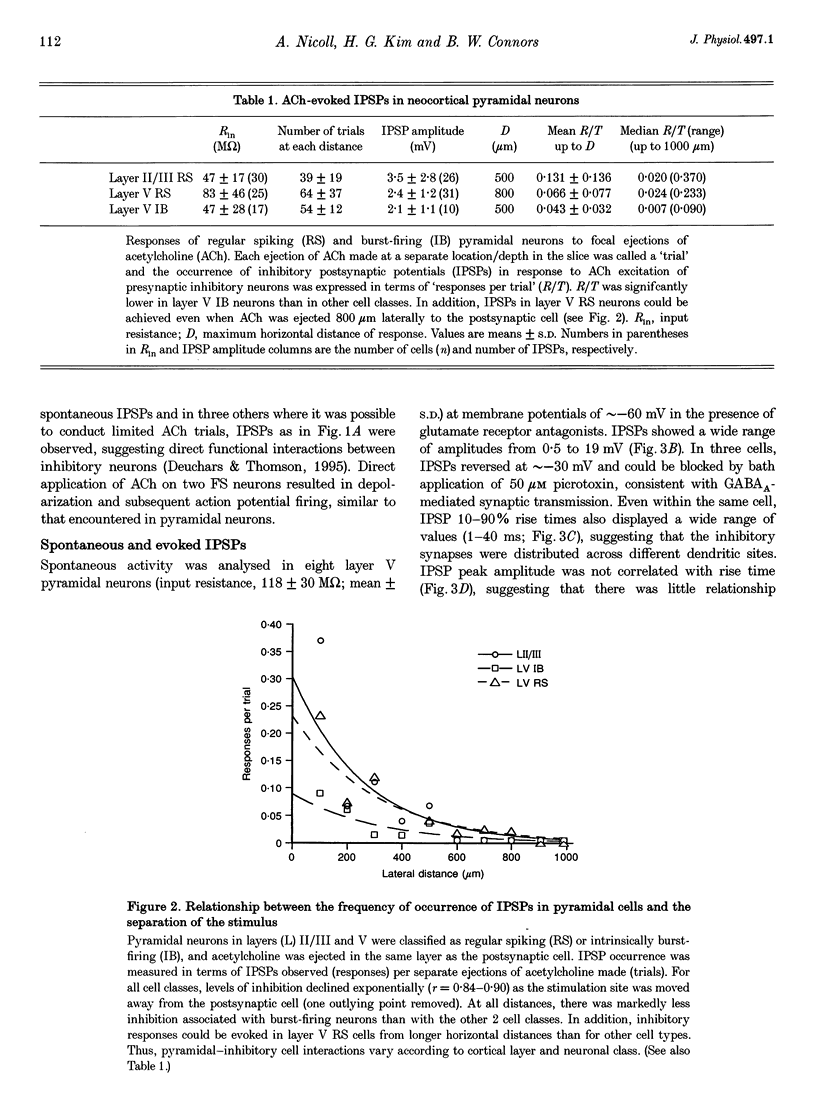
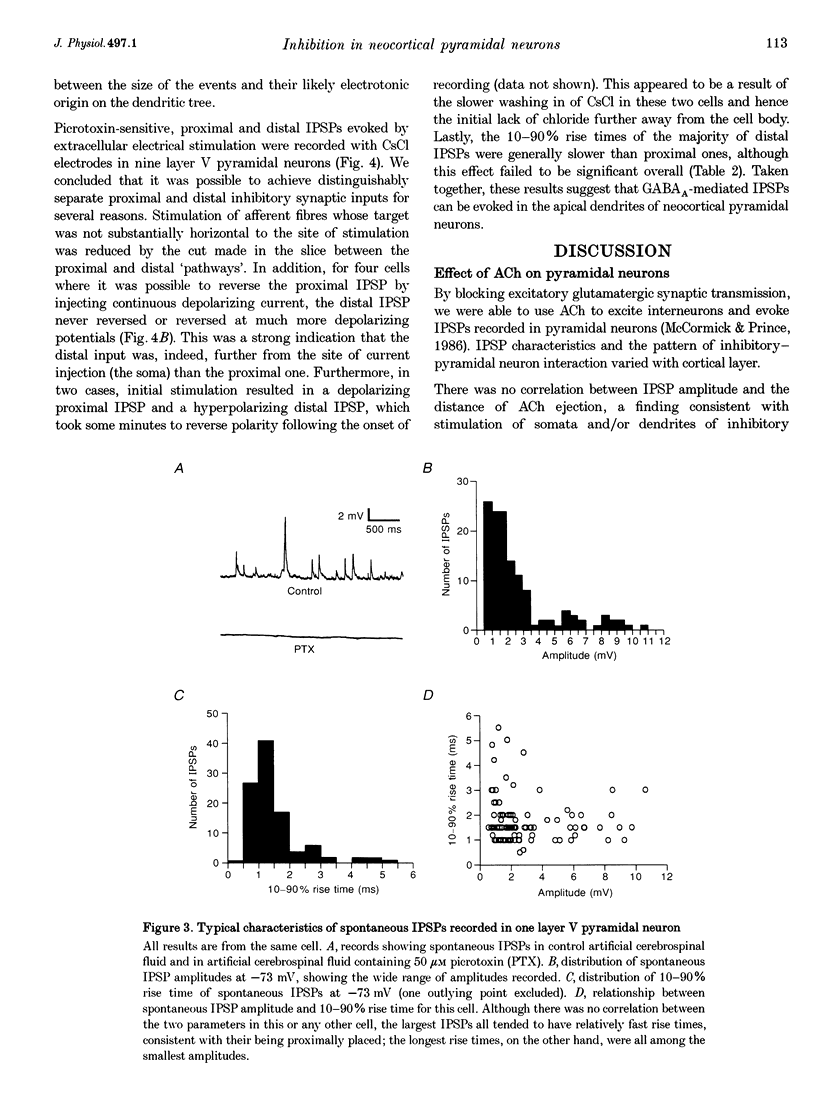
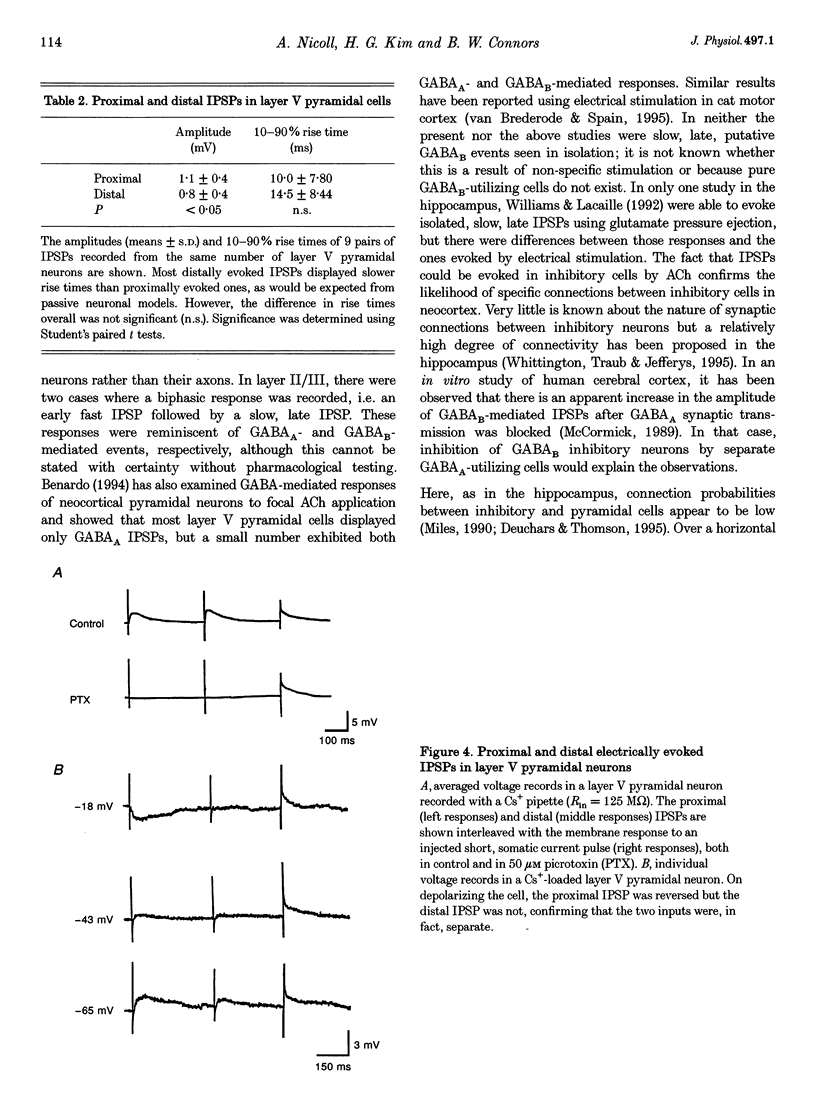
1. Inhibitory neuron-pyramidal cell interactions were investigated in slices of rat somatosensory cortex in which excitatory synaptic transmission was blocked with bath-applied glutamate receptor antagonists. Local inhibitory neurons were excited by focal pressure ejections of small (approximately 40 pl) volumes of 1-10 mM acetylcholine. 2. The frequency of inhibitory postsynaptic potentials (IPSPs) ("responses per trial' or R/T) declined as the stimulation distance was increased. Inhibitory inputs were most prevalent in layer II/III regular spiking (RS) pyramidal neurons (30 cells) where median R/T was 0.020. In layer V, the median R/T was 0.024 for RS neurons (25 cells), but significantly lower for burst-firing (IB) neurons (17 cells), where median R/T was 0.007 (P = 0.039). 3. IPSPs in individual layer V pyramidal cells were recorded with CsCl electrodes. In eight neurons, spontaneous picrotoxin-sensitive IPSPs were recorded and found to display a wide range of 10-90% rise times (1-34 ms), not correlated with amplitude (0.2-18 mV). For a further ten pyramidal neurons, extracellular stimulating electrodes were placed simultaneously in layers II/III and V/VI in order to evoke pairs of IPSPs whose waveforms were averaged and compared. In seven cells, IPSPs evoked from layer II/III (distal location) had longer 10-90% rise times than IPSPs evoked from layer V/VI stimulating electrodes (proximal location). In addition, "proximal' IPSPs could always be reversed by membrane depolarization whereas "distal' ones could not (n = 4/4). 4. This study showed that pyramid cell-inhibitory neuron interconnections are extensive but their spatial organization varies with cell class and with cortical layer. In addition, pyramidal neurons can receive inhibitory inputs from locations on their apical dendrites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benardo L. S. Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro. J Physiol. 1994 Apr 15;476(2):203–215. doi: 10.1113/jphysiol.1994.sp020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol. 1989 Apr;61(4):747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989 Nov;62(5):1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Chmielowska J., Stewart M. G., Bourne R. C., Hamori J. gamma-Aminobutyric acid immunoreactivity in mouse barrel field: a light microscopical study. Brain Res. 1986 Mar 19;368(2):371–374. doi: 10.1016/0006-8993(86)90584-6. [DOI] [PubMed] [Google Scholar]
- Connors B. W. GABAA- and GABAB-mediated processes in visual cortex. Prog Brain Res. 1992;90:335–348. doi: 10.1016/s0079-6123(08)63621-3. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars J., Thomson A. M. Single axon fast inhibitory postsynaptic potentials elicited by a sparsely spiny interneuron in rat neocortex. Neuroscience. 1995 Apr;65(4):935–942. doi: 10.1016/0306-4522(95)00020-j. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Characteristics of long-duration inhibitory postsynaptic potentials in rat neocortical neurons in vitro. Cell Mol Neurobiol. 1987 Mar;7(1):1–18. doi: 10.1007/BF00734986. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995 Apr;15(4):2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. G., Beierlein M., Connors B. W. Inhibitory control of excitable dendrites in neocortex. J Neurophysiol. 1995 Oct;74(4):1810–1814. doi: 10.1152/jn.1995.74.4.1810. [DOI] [PubMed] [Google Scholar]
- Kim H. G., Connors B. W. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993 Dec;13(12):5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991 Feb;65(2):247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986 Jun;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C. T., Burkhalter A. Organization of long-range inhibitory connections with rat visual cortex. J Neurosci. 1993 Feb;13(2):768–781. doi: 10.1523/JNEUROSCI.13-02-00768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Gilbert C. D., Rivlin P. K., Wiesel T. N. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991 Mar 15;305(3):370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Miles R., Tóth K., Gulyás A. I., Hájos N., Freund T. F. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996 Apr;16(4):815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Miles R. Variation in strength of inhibitory synapses in the CA3 region of guinea-pig hippocampus in vitro. J Physiol. 1990 Dec;431:659–676. doi: 10.1113/jphysiol.1990.sp018353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Inhibitory role of dentate hilus neurons in guinea pig hippocampal slice. J Neurophysiol. 1990 Jul;64(1):46–56. doi: 10.1152/jn.1990.64.1.46. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992 Jan;67(1):227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Peters A., Kara D. A. The neuronal composition of area 17 of rat visual cortex. II. The nonpyramidal cells. J Comp Neurol. 1985 Apr 8;234(2):242–263. doi: 10.1002/cne.902340209. [DOI] [PubMed] [Google Scholar]
- Peters A., Payne B. R., Josephson K. Transcallosal non-pyramidal cell projections from visual cortex in the cat. J Comp Neurol. 1990 Dec 1;302(1):124–142. doi: 10.1002/cne.903020110. [DOI] [PubMed] [Google Scholar]
- Salin P. A., Prince D. A. Electrophysiological mapping of GABAA receptor-mediated inhibition in adult rat somatosensory cortex. J Neurophysiol. 1996 Apr;75(4):1589–1600. doi: 10.1152/jn.1996.75.4.1589. [DOI] [PubMed] [Google Scholar]
- Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res. 1990 Mar 12;511(1):163–164. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Kisvárday Z. F., Martin K. A., Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience. 1983 Oct;10(2):261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- Weliky M., Kandler K., Fitzpatrick D., Katz L. C. Patterns of excitation and inhibition evoked by horizontal connections in visual cortex share a common relationship to orientation columns. Neuron. 1995 Sep;15(3):541–552. doi: 10.1016/0896-6273(95)90143-4. [DOI] [PubMed] [Google Scholar]
- White E. L., Amitai Y., Gutnick M. J. A comparison of synapses onto the somata of intrinsically bursting and regular spiking neurons in layer V of rat SmI cortex. J Comp Neurol. 1994 Apr 1;342(1):1–14. doi: 10.1002/cne.903420102. [DOI] [PubMed] [Google Scholar]
- Whittington M. A., Traub R. D., Jefferys J. G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995 Feb 16;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Williams S., Lacaille J. C. GABAB receptor-mediated inhibitory postsynaptic potentials evoked by electrical stimulation and by glutamate stimulation of interneurons in stratum lacunosum-moleculare in hippocampal CA1 pyramidal cells in vitro. Synapse. 1992 Jul;11(3):249–258. doi: 10.1002/syn.890110309. [DOI] [PubMed] [Google Scholar]
- van Brederode J. F., Spain W. J. Differences in inhibitory synaptic input between layer II-III and layer V neurons of the cat neocortex. J Neurophysiol. 1995 Sep;74(3):1149–1166. doi: 10.1152/jn.1995.74.3.1149. [DOI] [PubMed] [Google Scholar]


