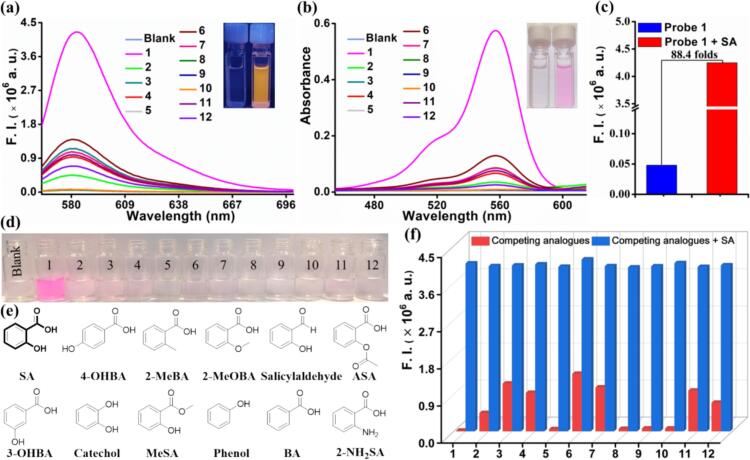
Fig. 1.
(a) Fluorescence and (b) absorption spectra of probe 1 (10 μM) were recorded after the addition of SA and its analogs (50 μM) at λex = 559 nm, using slits of 2/2 nm, and EtOH−H2O (7:3, v/v) as the solvent: blank, (1) SA, (2) 4-OHBA, (3) 2-MeBA, (4) 2-MeOBA, (5) salicylaldehyde, (6) ASA, (7) 3-OHBA, (8) catechol, (9) MeSA, (10) phenol, (11) BA, and (12) 2-NH2BA. Insert: photographs of UV light and sunlight of probe 1 in the absence or presence of SA. (c) Fluorescence enhancement ratio of the probe 1 upon the addition of SA. (d) Photographs of probe 1 (10 μM) after the addition of SA (50 μM) and its analogs. (e) The chemical structures of SA and its analogs. (f) The fluorescence intensity changes of probe 1 (10 μM) toward analogs (50 μM) at 582 nm without (red bar) or with (blue bar) 50 μM SA: (1) SA, (2) 4-OHBA, (3) 2-MeBA, (4) 2-MeOBA, (5) salicylaldehyde, (6) ASA, (7) 3-OHBA, (8) catechol, (9) MeSA, (10) phenol, (11) BA, and (12) 2-NH2BA in EtOH-H2O (7:3, v/v). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)