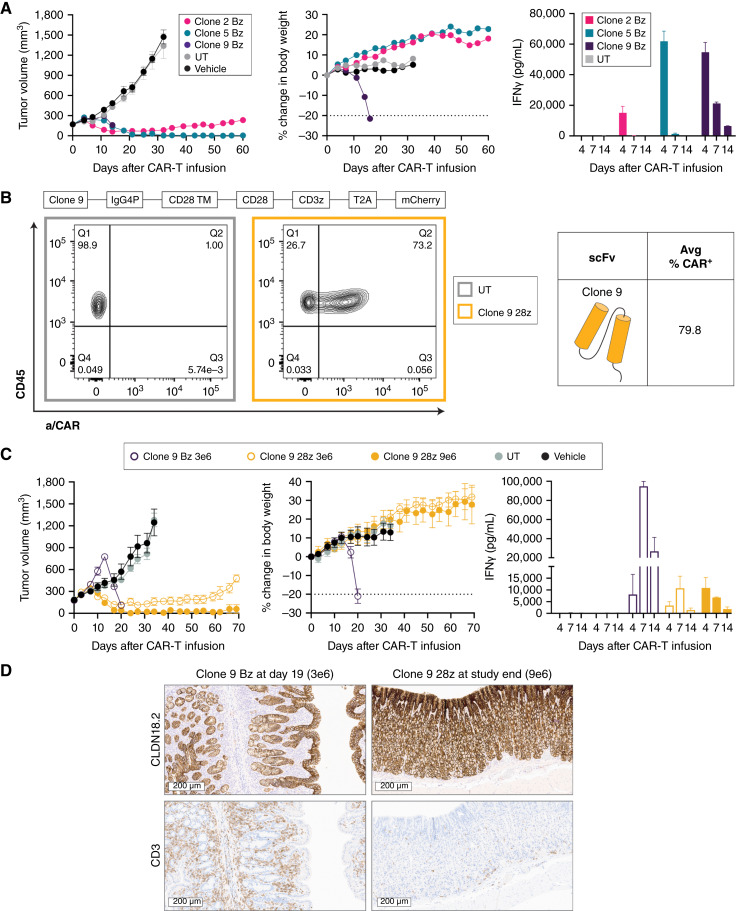
Figure 3.
Evaluation of CLDN18.2-targeting CAR-T cells in vivo. A, NSG mice bearing PaTu8988s HS xenografts (CLDN18.2 H-score = 268) were dosed by tail vein with 9e6 CAR+ CLDN18.2 Bz CAR-T cells; total T-cell infusion number was matched across groups. Tumor volume and body weight were measured biweekly (n = 9). Serum levels of IFNγ were measured at 4, 7, and 14 days after infusion (n = 3). B, Schematic representation of a second-generation CAR-T design modified to replace 4-1BB with a CD28 costimulatory domain (28z). The average transduction efficiency (CAR+, day 9) of multiple healthy donors for clone 9 28z is shown. Representative FC plots of CAR surface expression at day 9 after lentivirus transduction were compared with UT control for a single donor. C, NSG mice bearing PaTu8988s HS xenografts were dosed as described in A with clone 9 CD28z or Bz CAR-T (n = 6) at indicated doses. Serum levels of IFNγ were measured at 4, 7, and 14 days after infusion (n = 3). D, Representative images of CLDN18.2 (top row) and CD3 (bottom row) staining in the stomachs of mice dosed with clone 9 CAR-T cells from C at indicated time points. All data represent mean ± SEM of replicate experiments or animals.