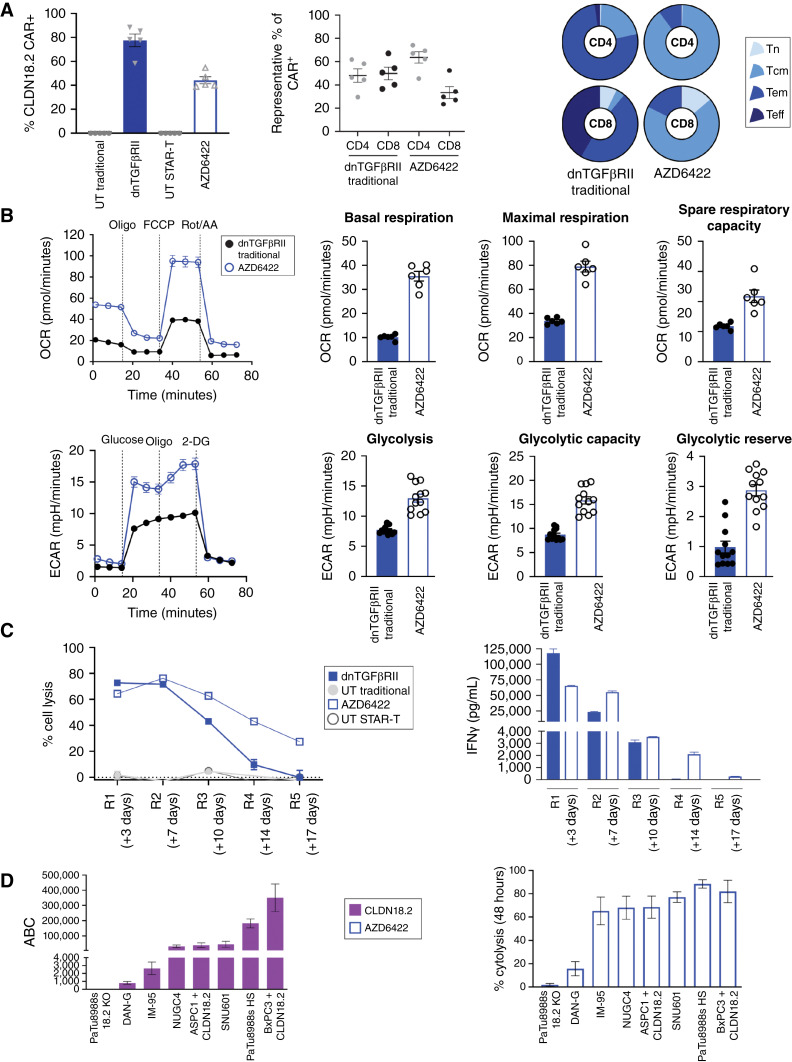
Figure 5.
Optimized manufacturing protocol, STAR-T, for the generation of the CAR-T product. A, Baseline characteristics of donor-matched dnTGFβRII CAR-T cells with traditional manufacture (day 10) vs. AZD6422 (day 4), including CAR+ expression, percent CD4 and CD8 expression, and T-cell phenotypic status as determined by cell surface expression of CCR7 and CD45RO. Results are shown for naïve (CCR7+/CD45RO−, Tn), central memory (CCR7+/CD45RO+, Tcm), effector memory (CCR7−/CD45RO+, Tem), and effector (CCR7−/CD45RO−, Teff) cells. Data are shown as mean ± SEM of representative donors. B, Comparison of bioenergetic profiles of traditionally manufactured dnTGFβRII CAR-T cells vs. AZD6422. Spare respiratory capacity was determined as the differential between basal and maximum respiration. 2-DG, 2-deoxy-D-glucose; ECAR, extracellular acidification rate; FCCP, carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone; OCR, oxygen consumption rate; Oligo, oligomycin; Rot/AA, rotenone and antimycin A. C, Serial restimulation assay to examine cytotoxicity and persistence of dnTGFβRII CAR-T cells and AZD6422. CAR-T cells were co-cultured at a ratio of 1:2 with BXPC3 + CLDN18.2, tumor lysis was measured every 3 to 4 days, and IFNγ was profiled at 24 hours after each new co-culture. Representative of multiple donors. D, Results of quantitative FC to determine cell surface expression of CLDN18.2 across multiple cancer cell lines. Percent cytolysis was determined by xCELLigence RTCA assay after 48 hours of co-culture with AZD6422 at an E:T ratio of 1:1. Data represent mean ± SEM of replicate experiments.