Abstract
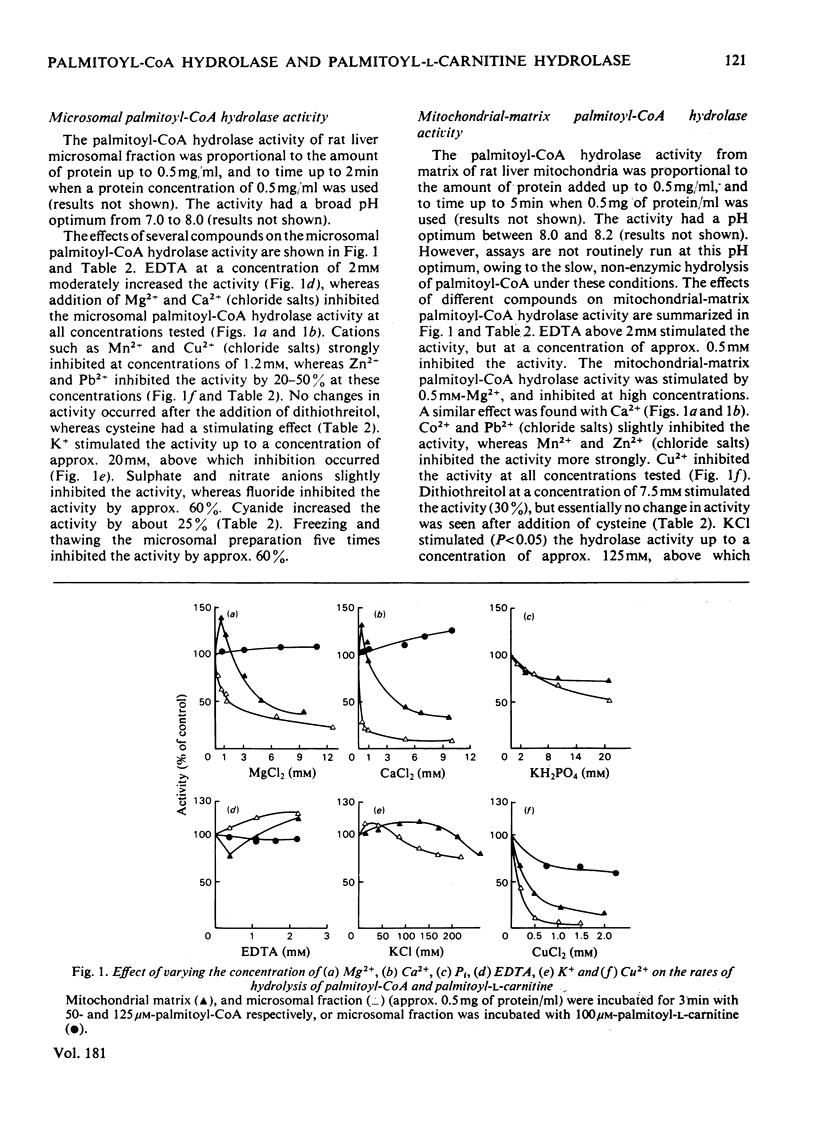
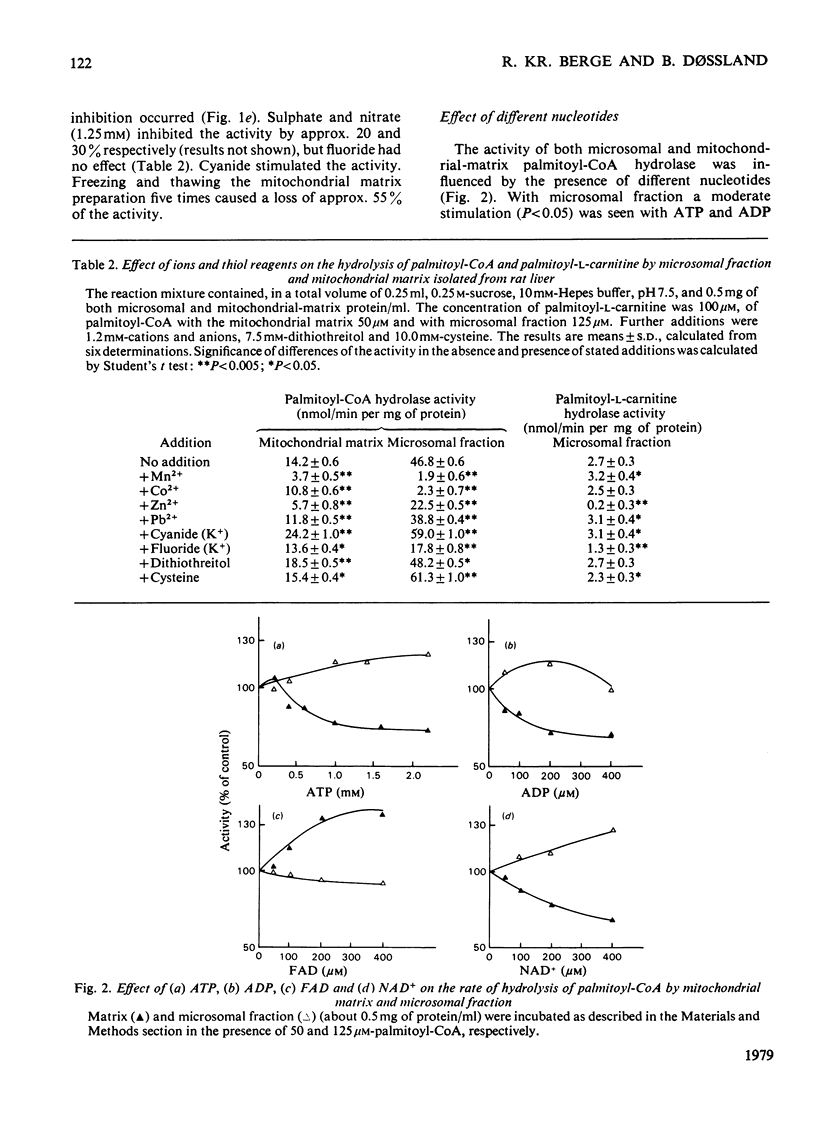
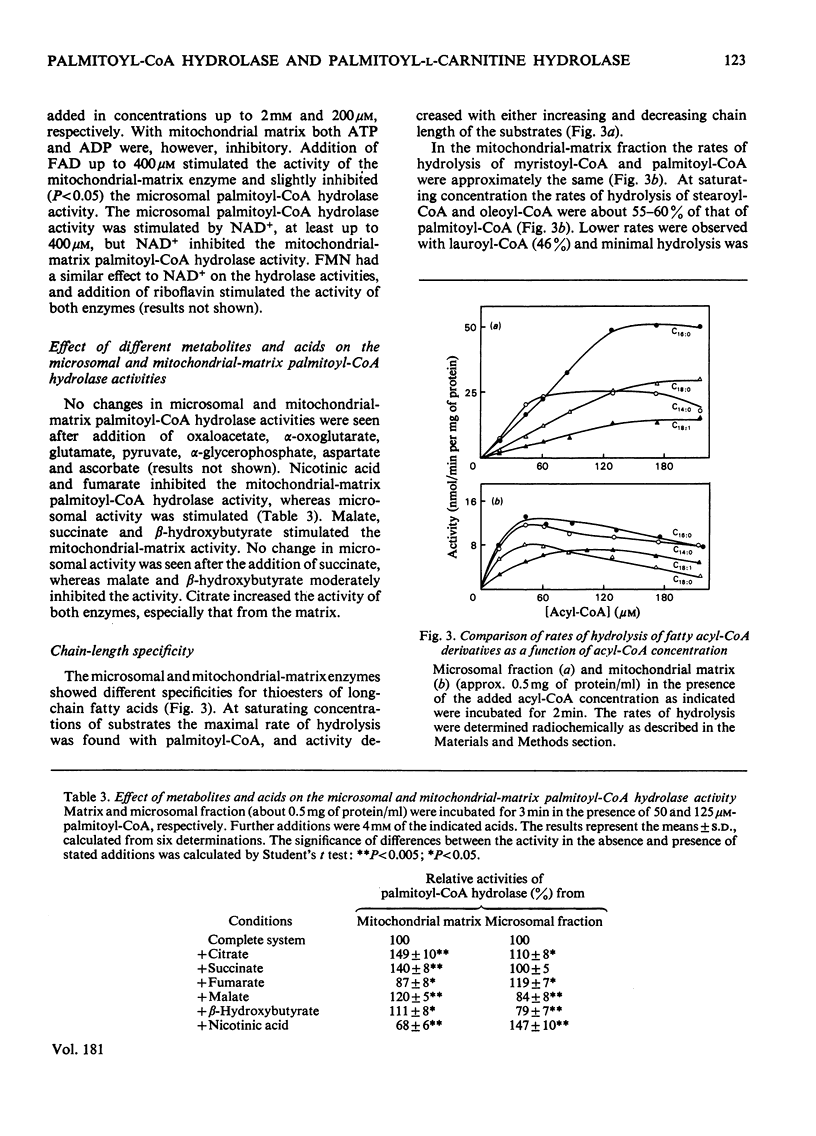
Palmitoyl-CoA hydrolase (EC 3.1.2.2) and palmitoyl-L-carnitine hydrolase (EC 3.1.1.28) activities from rat liver were investigated. 1. Microsomal and mitochondrial-matrix palmitoyl-CoA hydrolase activities had similar pH and temperature optima, although the activities showed different temperature stability. They were inhibited by Pb2+ and Zn2+. The palmitoyl-CoA hydrolase activities in microsomal fraction and mitochondrial matrix were differently affected by the addition of Mg2+, Ca2+, Co2+, K+ and Na+ to the reaction mixture. ATP, ADP and NAD+ stimulated the microsomal activity and inhibited the mitochondrial-matrix enzyme. The activity of both the microsomal and mitochondrial-matrix hydrolase enzymes was specific for long-chain fatty acyl-CoA esters (C12-C18), with the highest activity for palmitoyl-CoA. The apparent Km for palmitoyl-CoA was 47 microM for the microsomal enzyme and 17 microM for the mitochondrial-matrix enzyme. 2. The palmitoyl-CoA hydrolase and palmitoyl-L-carnitine hydrolase activities of microsomal fraction had similar pH optima and were stimulated by dithiothreitol, but were affected differently by the addition of Pb2+, Mg2+, Ca2+, Mn2+ and cysteine. The two enzymes had different temperature-sensitivities. 3. The data strongly suggest that palmitoyl-CoA hydrolase and palmitoyl-L-carnitine hydrolase are separate microsomal enzymes, and that the hydrolysis of palmitoyl-CoA in the microsomal fraction and mitochondria matrix was catalysed by two different enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Barber E. D., Lands W. E. Determination of acyl-CoA concentrations using pancreatic lipase. Biochim Biophys Acta. 1971 Nov 13;250(2):361–366. doi: 10.1016/0005-2744(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Barden R. E., Cleland W. W. 1-Acylglycerol 3-phosphate acyltransferase from rat liver. J Biol Chem. 1969 Jul 10;244(13):3677–3684. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H., Hülsmann W. C. Long-chain acyl-CoA hydrolase activity in serum: identity with clearing factor lipase. Biochim Biophys Acta. 1973 Jan 19;296(1):241–248. doi: 10.1016/0005-2760(73)90064-7. [DOI] [PubMed] [Google Scholar]
- Jansen H., Oerlemans M. C., Hülsmann W. C. Differential release of hepatic lipolytic activities. Biochem Biophys Res Commun. 1977 Aug 8;77(3):861–867. doi: 10.1016/s0006-291x(77)80057-0. [DOI] [PubMed] [Google Scholar]
- Jansen H., van Zuylen-van WIGGEN A., Hülsmann W. C. Lipoprotein lipase from heart and liver: an immunological study. Biochem Biophys Res Commun. 1973 Nov 1;55(1):30–37. doi: 10.1016/s0006-291x(73)80055-5. [DOI] [PubMed] [Google Scholar]
- Jezyk P. F., Hughes H. N. Studies on the hydrolysis and utilization of long chain acyl CoA thioesters by liver microsomes. Lipids. 1971 Feb;6(2):107–112. doi: 10.1007/BF02531325. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Steinberg D. Hormone-sensitive triglyceride lipase from rat adipose tissue. Methods Enzymol. 1975;35:181–189. doi: 10.1016/0076-6879(75)35154-9. [DOI] [PubMed] [Google Scholar]
- Kurooka S., Hosoki K., Yoshimura Y. Some properties of long fatty acyl-coenzyme A thioesterase in rat organs. J Biochem. 1972 Apr;71(4):625–634. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahadevan S., Sauer F. Carnitine ester hydrolase of rat liver. J Biol Chem. 1969 Aug 25;244(16):4448–4453. [PubMed] [Google Scholar]
- McMurray W. C., Magee W. L. Phospholipid metabolism. Annu Rev Biochem. 1972;41(10):129–160. doi: 10.1146/annurev.bi.41.070172.001021. [DOI] [PubMed] [Google Scholar]
- PORTER J. W., LONG R. W. A study of the role of palmityl coenzyme A in fatty acid synthesis by the pigeon liver system. J Biol Chem. 1958 Jul;233(1):20–25. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L. Effect of long-chain fatty acids and acyl-CoA on mitochondrial permeability, transport, and energy-coupling processes. J Bioenerg Biomembr. 1976 Dec;8(6):293–311. doi: 10.1007/BF00765158. [DOI] [PubMed] [Google Scholar]


