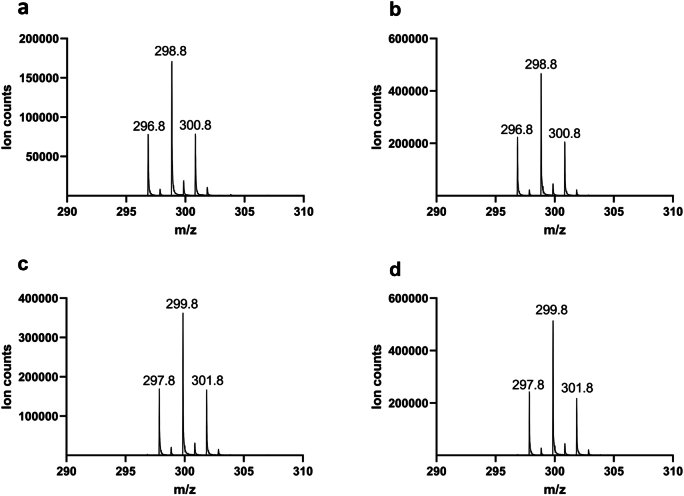
Extended Data Fig. 5. Mass spectra of the nitrile product when AetD reaction was performed with 13C-labelled isotopologues of 5,7-dibromo-L-tryptophan.
a, AetD + 5,7-dibromo-L-tryptophan. b, AetD + [2-13C1]-5,7-dibromo-L-tryptophan. c, AetD + [3-13C1]-5,7-dibromo-L-tryptophan. d, AetD + [1,2,3-13C3]-5,7-dibromo-L-tryptophan. A 1 Da increase in the mass of the nitrile product was observed only when [3-13C1]-5,7-dibromo-L-tryptophan and [1,2,3-13C3]-5,7-dibromo-L-tryptophan were used as substrates, confirming the β-carbon C-3 is retained in the product.