FIG. 7.
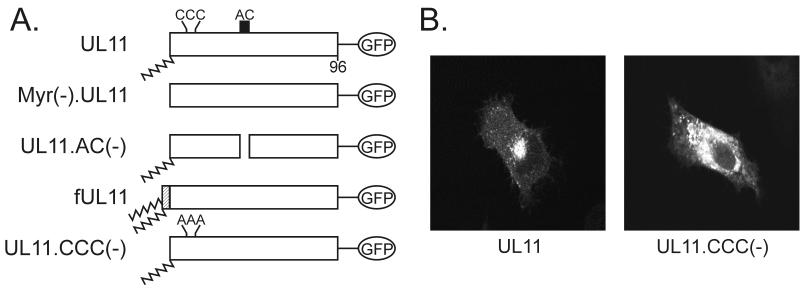
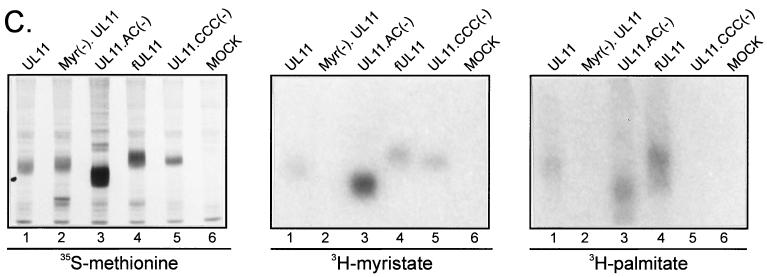
Palmitylation of UL11. (A) Mutational analysis of the CCC motif. The wild-type UL11 protein (top) is myristylated (wavy line) and contains the acidic cluster (AC) (residues 37 to 43, indicated by a black box) along with a cluster of three cysteines (residues 11 to 13). An N-terminal 10-amino-acid sequence from the Fyn protein (hatched box) is known to be sufficient for both myristylation and palmitylation (double wavy line). All constructs have the GFP protein (open oval) fused to their C termini. (B) Subcellular localization. The indicated constructs were transfected into COS-1 cells, and 18 h later, the UL11-GFP chimeras were visualized by confocal microscopy. (C) Biochemical analysis. Transfected COS-1 cells were labeled with either [35S]methionine for 2.5 h, [3H]myristic acid for 10 min, or [3H]palmitic acid for 60 min. Cell lysates were prepared, and UL11-GFP proteins were immunoprecipitated, mixed with sample buffer (without β-mercaptoethanol), resolved by SDS-PAGE, and visualized by autoradiography.