Abstract
Boston Children’s Hospital has established a genomic sequencing and analysis research initiative to improve clinical care for pediatric rare disease patients. Through the Children’s Rare Disease Collaborative (CRDC), the hospital offers CLIA-grade exome and genome sequencing, along with other sequencing types, to patients enrolled in specialized rare disease research studies. The data, consented for broad research use, are harmonized and analyzed with CRDC-supported variant interpretation tools. Since its launch, 66 investigators representing 26 divisions and 45 phenotype-based cohorts have joined the CRDC. These studies enrolled 4653 families, with 35% of analyzed cases having a finding either confirmed or under further investigation. This accessible and harmonized genomics platform also supports additional institutional data collections, research and clinical, and now encompasses 13,800+ patients and their families. This has fostered new research projects and collaborations, increased genetic diagnoses and accelerated innovative research via integration of genomics research with clinical care.
Subject terms: Paediatric research, Genetics research
Introduction
The availability and efficiency of genomic sequencing in the diagnosis of rare monogenic diseases has led to frequent use of genomics in research and gradual adoption in clinical practice. Over the past decade, numerous large rare disease sequencing research studies have characterized the burden of Mendelian disease across various phenotypes1–4. The clinical impact of timely genomic sequencing for pediatric patients has been well-established in the critical care setting5–8. However, another advancement has been the development of genomics-driven platforms that facilitate research-informed healthcare for broader groups of patients9–14 in a variety of care contexts. Critically, research-clinical integration for genomics includes building infrastructure to consent for research sequencing, confirm selected research findings and deliver clinically validated results directly to patients, as well as a mechanism to conduct deeper ongoing analysis in a research setting on non-diagnostic clinical cases. Establishment of a research-clinical cycle leverages the power of research to improve clinical care by filling gaps in diagnostics and accessibility while maintaining the highest standards for clinical diagnosis.
Exome sequencing (ES) has had broad use in rare disease research studies and is increasingly utilized in clinical care. However, genome sequencing (GS) has become more common as the cost of sequencing has decreased. GS has been shown to increase diagnostic yields up to 10% through improved variant calling for small variants, copy number variants (CNVs), and other structural variants (SVs) and the ability to interrogate the non-coding space15–18 including in regions of known disease-associated genes. In addition, other genomic technologies, such as long-read GS and transcriptome sequencing, have been deployed to resolve cases undiagnosed by ES/GS19–22. However, these other technologies are still mostly limited to research.
The Children’s Rare Disease Collaborative (CRDC) at Boston Children’s Hospital (BCH) was launched in 2018 with the goal of integrating research and clinical genomic data into an accessible genomics platform to drive pediatric precision medicine. Phase I, completed in 2019, included the establishment of an infrastructure for consenting patients and their parents, data sharing, and analysis of ES for 1046 affected individuals across 15 disease cohorts11. Here, we describe the results from Phase II and the first five years overall of the CRDC. Having established a large cohort of patients with pediatric rare disease presentations ascertained by subspecialty experts, with deep disease-specific phenotype information and genomic data, we demonstrate the potential of an institutional research-clinical partnership in facilitating new discoveries and advancing pediatric healthcare.
Results
Establishing a genomic sequencing ecosystem
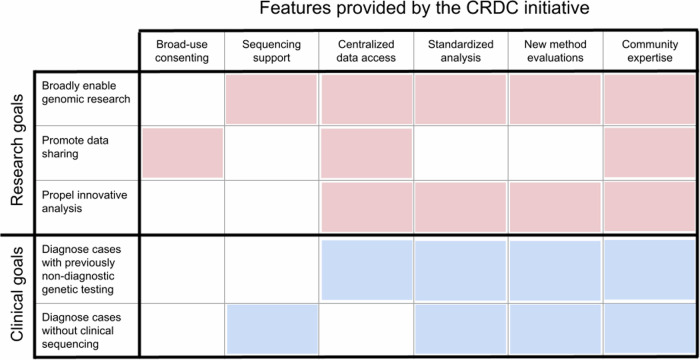
As previously described, the CRDC was created in alignment with our institutional goals as part of the BCH Research Strategic plan and as the outcome of a Blue Ribbon committee commissioned in 201811. The goal was to establish a scalable, clinical-grade genomic sequencing platform that advances rare disease research and improves clinical care. To this end, the collaborative developed a variety of features and resources for research and clinical communities across the institution (Fig. 1). These features include integrating language for broad-use research and data sharing into consents, extensive financial support for research ES and GS, centralized data access to both research- and clinically-generated sequencing data, a comprehensive and standardized analysis platform, a mechanism to evaluate novel methods and analytics, and a network of investigators with diverse disease-specific expertise. The establishment of these resources has enabled investigators at all career levels to perform genomic research, promoted data sharing internally and externally, propelled implementation of innovative analysis and provided access to new pathways of diagnosis for individuals not able to obtain clinical testing or that had received nondiagnostic results.
Fig. 1. Key features of the CRDC.
This chart displays the six key features of the CRDC across the top and how they contribute to its research (red) and clinical (blue) goals. This figure was created in Microsoft PowerPoint.
The CRDC began offering research genomic sequencing for selected rare disease cohorts in late 2018; in the first year (Phase I), the collaborative generated ES data for 1046 affected individuals across 15 cohorts, developed consent language for broad-use research and implemented a harmonized data processing and standardized analysis platform11. Since then, additional disease cohorts were selected for funding about once a year via a hospital-wide call for applications. Cohorts were chosen based on potential for novel discoveries and scientific innovation. Moreover, the selection criteria were inclusive, with a goal to broaden the availability of genomic sequencing across all the divisions and departments and investigators at all career levels. As of early 2024, the CRDC included 45 disease cohorts led by 66 investigators from 26 departments/programs (Table 1). These disease cohorts each covered at least 5 and up to hundreds of different genetic diseases, defined by the Genetic and Rare Diseases Information Center (GARD, rarediseases.info.nih.gov).
Table 1.
Overview of the disease cohorts involved in the CRDC
| Department/Division | Disease cohort | Families with genomic data and consent for broad-use research | Median age of patient at enrollment (years)a | Percent that includes both parents (trio sequencing)a | Percent of probands with GSa | Average number of HPO termsa,b | Overall number of families with research genomic data |
|---|---|---|---|---|---|---|---|
| Neurology | Unexplained Epilepsies | 941 | 9.4 | 66% | 36% | 51 | 1166 |
| Cerebral Palsy and Related Disorders | 265 | 8.7 | 55% | 13% | 75 | 265 | |
| Hereditary Spastic Paraplegia and Movement Disorders | 68 | 10.0 | 71% | 93% | 84 | 68 | |
| Brain Malformations | 43 | 4.6 | 77% | 33% | 68 | 469 | |
| Cerebrovascular Disorders | 21 | 11.4 | 52% | 0% | 47 | 21 | |
| Agenesis of the Corpus Callosum | 0 | - | - | - | - | 24 | |
| Genetics and Genomics | Ultra-Rare Disease | 425 | 7.4 | 69% | 45% | 73 | 1235 |
| ADHD and Related Disorders | 339 | 10.3 | 72% | 7% | 46 | 339 | |
| Myopathies and Dystrophies | 57 | 10.6 | 83% | 22% | 64 | 456 | |
| Sudden Unexpected Death in Childhood (SUDP/SIDS) | 26 | 0.6 | 50% | 65% | - | 486 | |
| Cornelia de Lange Syndrome and Related Disorders | 7 | 10.5 | 43% | 0% | 103 | 7 | |
| Interstitial Cystitis | 0 | - | - | - | - | 354 | |
| Endocrinology | Idiopathic Short Stature | 91 | 9.3 | 56% | 2% | 56 | 91 |
| Connective Tissue Disorders | 45 | 15.6 | 31% | 20% | 85 | 45 | |
| Osteogenesis Imperfecta | 38 | 13.8 | 61% | 0% | 51 | 38 | |
| Disorders of Sex Development and Hypospadias | 33 | 3.7 | 48% | 3% | 43 | 169 | |
| Precocious Puberty | 28 | 9.5 | 25% | 0% | 43 | 28 | |
| Cancer and Blood Disorders | Anemias and Iron Disorders | 26 | 8.4 | 58% | 5% | 29 | 332 |
| Bone Marrow Failure and Leukemia Predisposition | 20 | 3.7 | 65% | 90% | 70 | 282 | |
| Schwamman Diamond Syndrome | 3 | - | 33% | 100% | 91 | 3 | |
| Sickle Cell Disease | 0 | - | - | - | - | 974 | |
| Gastrointestinal | Inflammatory Bowel Disease | 793 | 15.4 | 24% | 37% | 41 | 811 |
| Congenital Diarrheas and Enteropathies | 94 | 6.6 | 22% | 30% | 57 | 106 | |
| Intestinal Failure due to Malrotation and Volvulus | 13 | 6.3 | 62% | 100% | 40 | 13 | |
| Otorhinolaryngology | Hearing Loss | 451 | 7.2 | 53% | 13% | 43 | 451 |
| Hearing Loss and Cochlear Implants | 62 | 2.4 | 3% | 45% | 41 | 62 | |
| Peripheral Vestibular Disorders | 59 | 13.4 | 44% | 2% | 62 | 59 | |
| Immunology | Immunodeficiencies, Autoimmunity and Immune Dysregulation | 291 | 10.8 | 44% | 8% | 67 | 372 |
| Severe Pediatric COVID-19 and MIS-C | 132 | 8.3 | 1% | 80% | 55 | 150 | |
| Graves disease | 31 | 17.5 | 0% | 29% | 64 | 31 | |
| Pulmonology | Interstitial Lung Disease | 220 | 10.7 | 35% | 26% | 65 | 220 |
| Bronchiectasis | 148 | 20.7 | 11% | 37% | 64 | 148 | |
| Urology | Bladder Exstrophy-Epispadias Complex | 87 | 9.1 | 41% | 21% | 45 | 104 |
| Disorders of Voiding | 12 | 16.5 | 25% | 0% | 55 | 12 | |
| Nephrology | Nephrotic Syndrome and Glomerular Disease | 45 | 9.7 | 9% | 56% | 49 | 251 |
| Urinary Tract Stone Disease | 14 | 5.4 | 86% | 29% | 58 | 14 | |
| Psychiatry | Early-Onset Major Depression | 30 | 13.1 | 30% | 33% | 53 | 30 |
| Early-Onset Psychosis | 7 | 14.6 | 14% | 0% | 51 | 7 | |
| Ophthalmology | Infantile Esotropia | 24 | 5.0 | 71% | 21% | 36 | 24 |
| Infantile Nystagmus | 6 | 6.3 | 67% | 17% | - | 6 | |
| Newborn Medicine | Neonatal Critical Illness | 16 | 0.2 | 31% | 63% | 67 | 16 |
| Complex Fetal Cases | 11 | fetal | 91% | 27% | 37 | 11 | |
| Intersectional | Congenital Heart Disease and Autism Spectrum Disorder | 24 | 11.1 | 75% | 67% | 81 | 24 |
| Anesthesiology | Severe Chronic Pain and Insensitivity to Pain | 22 | 15.1 | 68% | 9% | 52 | 22 |
| Plastic and Oral Surgery | Ectodermal Dysplasia and Cleft Lip or Palate | 1 | - | 0% | 0% | 119 | 1 |
HPO human phenotype ontology, SUDP sudden unexpected death in pediatrics, SIDS sudden infant death syndrome, ADHD attention deficit/hyperactivity disorder, MIS-C multisystem inflammatory syndrome in children.
aData for families sequenced through the CRDC with broad-use research consent.
bIncluded HPO terms collected by researchers and extracted from the electronic health record with Clinithink.
Since the launch of the collaborative, the process to onboard new cohorts has been streamlined, particularly at the stage of Institutional Review Board (IRB) review, which was 16% faster for the 15 most recent consents that include standardized CRDC-specific language than the first 12. There have been many improvements in the process of enrolling individuals and collecting samples. Efforts to develop methods of remote consenting and sample collection accelerated during the COVID-19 pandemic to mitigate pandemic-related restrictions on general on-site interactions with patients and research participants. The ability to consent and enroll remotely/electronically and to remotely collect buccal samples (ES only) continues even as clinics and research are permitted to occur on-site.
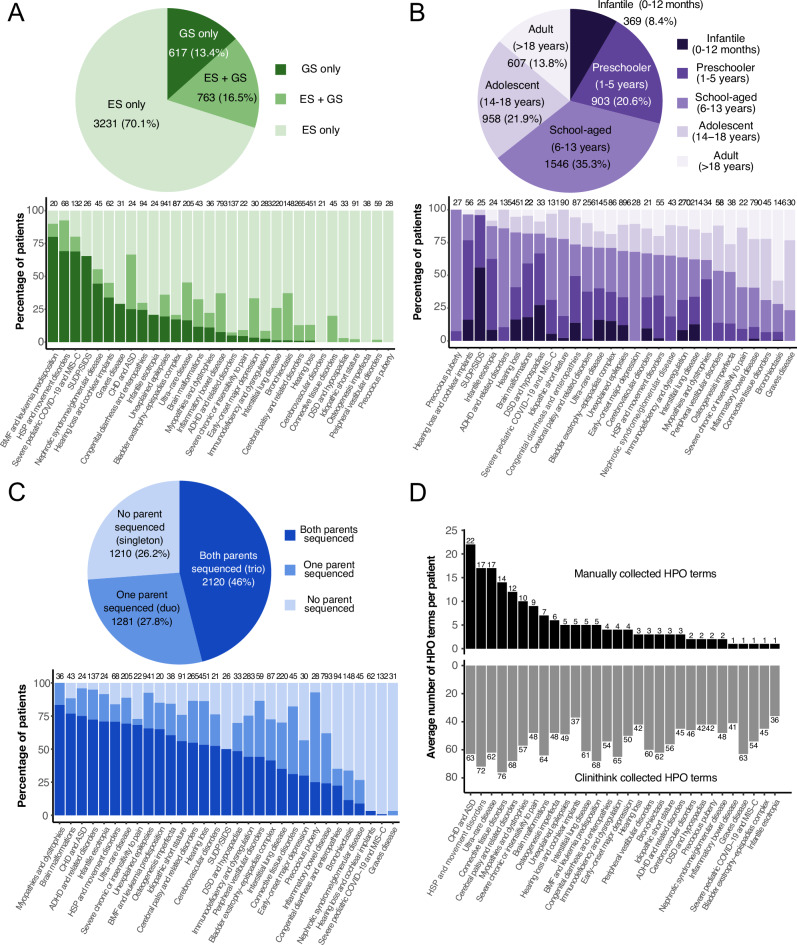
Additionally, in Phase II, the CRDC began supporting GS, in addition to ES, as a sequencing test. The original experimental design was to first perform ES on the proband and available family members and then reflex selected non-diagnostic cases to GS of the proband. However, in March 2022, GS began to be offered as a first-line test to all disease cohorts. Since then, usage of GS has grown, currently accounting for 35% of tests ordered. The majority of tests ordered continues to be ES, however, largely because buccal swabs and thus remote sample collection have only recently been accepted by GeneDx for GS. Overall, 70% of probands have only ES data while 17% of probands have ES + GS data and 13% have GS as the primary test (Table 1, Fig. 2A).
Fig. 2. Overview of participants and data included in the CRDC.
Overview of participants and data included in the CRDC. A Distribution of type of sequencing performed. B Distribution of age at enrollment. C Distribution of sequencing of parents. For (A–C), the pie chart includes patients for all CRDC-sequenced cohorts combined and the bar chart includes individual CRDC-sequenced cohorts with at least 20 patients. D Average number of HPO (Human Phenotype Ontology) terms collected per patient for individual CRDC-sequenced cohorts with at least 20 patients. Top: HPO terms collected manually by research teams. Bottom: HPO terms extracted from the electronic health record by Clinithink. SUDP sudden unexpected death in pediatrics, SIDS sudden infant death syndrome, ADHD attention deficit/hyperactivity disorder, DSD disorders of sex development, MIS-C multisystem inflammatory syndrome in children, HSP hereditary spastic paraplegia, ASD autism spectrum disorder, CHD congenital heart defect. This figure was created in R with ggplot39.
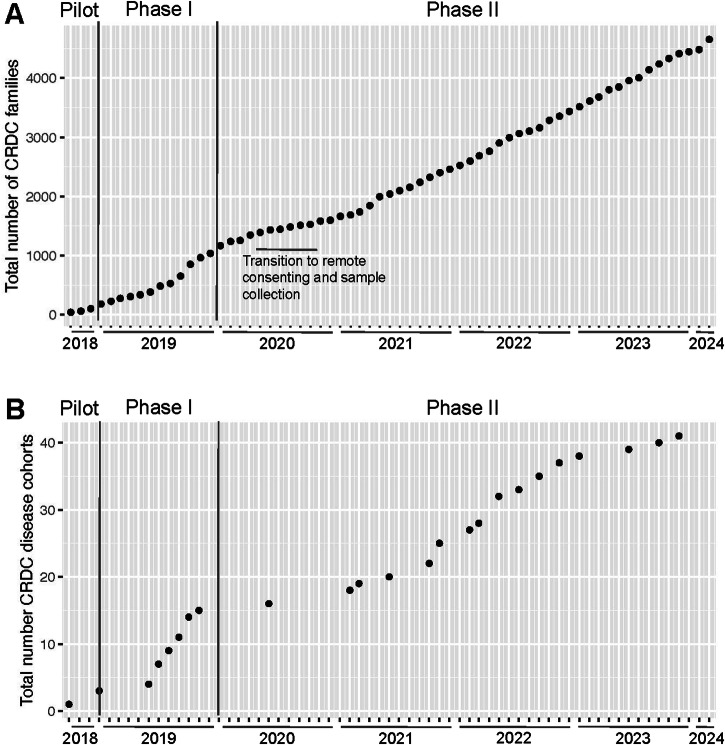
As of February 2024, 6308 rare disease patients and their families (13,723 individuals) that consented to a research study have had ES (100x average coverage) and/or GS (40× average coverage) performed via the CRDC, with 4653 of those families (11,150 individuals across 41 disease cohorts) consented for broad-use research purposes and data sharing (Fig. 3). These data have been harmonized in an institution-wide genomics repository with genomic and phenotypic data collected from other research projects and support sources. Additionally, a workflow was established whereby data generated from clinically-ordered sequencing was returned and harmonized in the repository, facilitating clinically-driven re-analysis and reflex to a research study. The repository thus contains 5694 families under a broad-use research consent, 4916 families under other research consents, and 3266 clinically-sequenced families not currently involved in a research study for a total of 31,168 individuals. All data were made available to the appropriate researchers or clinicians in a standardized genomics analysis platform with multiple tools for investigation including GeneDx’s Discovery Platform, Illumina’s Emedgene, a local instance of the Broad Institute’s Seqr platform23 and a gnomAD-like browser developed in-house, BCH Aggregator.
Fig. 3. Growth of the CRDC since launch.
These plots track the increase in total number of (A) families receiving genomic sequencing through the collaborative under a broad-use research consent and (B) disease cohorts enrolling such families. Enrollment slowed slightly in the early months of the COVID-19 pandemic as researchers transitioned to remote consenting and sample collection, as marked on the plot. This figure was created in R with ggplot39.
The set of CRDC-sequenced data consented for broad research (4,653 families) is described in this report and comprises data from probands who are mostly pediatric (86% with age ≤18 years at time of enrollment, median age = 11 years) (Table 1, Fig. 2B) and are 53% male and 47% female. Where possible, the biological parents of probands and other relevant family members were also consented to the study. Forty-six percent of families (2120) included both biological parents (trios) and another 28% included one biological parent (duos) (Fig. 2C). Ten percent of families included at least one other non-parental family member, 91% of whom included siblings. Seventy-two percent of CRDC probands were self–reported as White, non-Hispanic/non-Latine (compared to 67% of the overall hospital patient population)24.
Phenotypic information is collected in a centralized repository for studies involved in the CRDC via two methods. The first is manual entry of clinical information into disease-specific REDCap25,26 databases by individual research teams. Each disease cohort had an average of 1–16 Human Phenotype Ontology (HPO) terms per patient (Fig. 2D) and with an overall average across all cohorts of 5 HPO terms/patient. Many research groups also collected additional phenotype information (e.g., EEGs and MRIs) and the disease-specific REDCap databases could have hundreds of fields. The second method of phenotypic data collection is pulling from the electronic health record (EHR) via Clinithink (www.clinithink.com), a natural language processing algorithm. This method resulted in an average of 52 HPO terms per proband (Fig. 2D). All these data were then collected in a single central REDCap repository allowing for easy dissemination to various analysis tools.
Advancing rare disease research
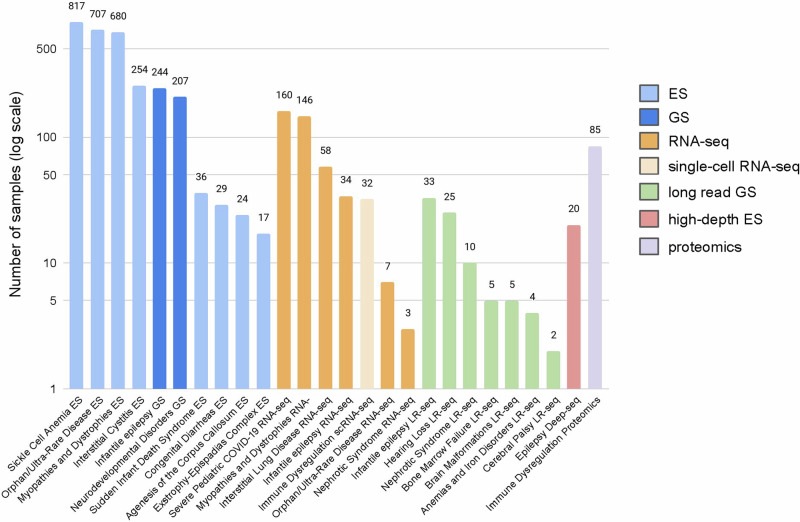
In addition to providing sequencing support for studies that prospectively enrolled patients under a broad-use research consent, the CRDC has also supported several projects with the goal of expanding access to sequencing and other diagnostic methods. One major arm of this was providing support for ES/GS and analysis for patients already enrolled in a different study under a non-broad-use research consent (i.e., allowing only for more limited sharing of data). 1846 patients (2771 individuals) have thus been sequenced across nine disease cohorts including sickle cell anemia, orphan/ultra-rare disease, myopathies/dystrophies, neurodevelopmental disorders, and interstitial cystitis27 (Fig. 4), resulting in over 100 additional diagnoses so far.
Fig. 4. Additional projects supported by the CRDC.
A number of samples were included in additional projects performed to increase access to sequencing (ES, GS) and evaluate orthogonal methods for identifying the genetic basis of a rare disease presentation: RNA-seq, single-cell RNA-seq (scRNA-seq), long-read genome sequencing (LR-seq), high-depth exome sequencing to detect somatic mosaic variants (Deep-seq) and proteomics. This figure was created in Microsoft Excel.
The other major arm was supporting pilot projects to investigate and implement orthogonal experimental methods for identifying the genetic/genomic basis of a rare disease presentation (Fig. 4). One such project involved performing RNA-seq on over 400 samples across seven different cohorts including myopathies, pulmonary disease, severe COVID-19 and epilepsy. Data analysis is ongoing but already a few solved cases have been supported by functional information gleaned from the transcriptomic data, particularly for the COVID-19 and myopathies cohorts, which performed RNA-seq on related tissues: blood and muscle, respectively. Another method investigated was long-read sequencing with data generated for over 80 patients from seven cohorts including hearing loss, epilepsy, and nephrotic syndrome. Preliminary analysis of the data from hearing loss patients has resulted in three additional pathogenic findings undetectable with short-read genome sequencing. Other ongoing projects include testing the utility of single-cell transcriptomics, high-depth exome sequencing for identifying somatic mutations and proteomics in solving rare diseases.
Another major component of this study was creating opportunities for collaboration both within and outside the institution (Table 2). Critical to this was the incorporation of broad sharing for research use into the consent forms. In addition to the 4653 families sequenced via the CRDC, 1041 additional patients and families received genomic sequencing under a consent that allowed for such broad sharing of data. The genomic and phenotypic data for these individuals are available in a de-identified format for cross-cohort analyses in custom in-house tools including BCH Aggregator and Cohort Family Analysis. BCH Aggregator is an integrated database of sequencing data and clinical phenotypes and has a web portal for visualization and exploration. This aggregate resource allows users to investigate how many individuals have variants at a specific locus of interest, which phenotypes those individuals have, the name of the investigator who originally consented the patients and it provides statistics for phenotype-specific burden tests of variants in genes. The Cohort Family Analysis tool aggregates candidate variants produced by family-based prioritization tools across families and cohorts, provides information on variant inheritance, participant phenotype, consenting investigator, allele frequencies, protein impact of variation, protein annotations and protein structures, variant interpretations, splicing, conservation and known gene-disease relationships, and includes various filtering options. Both of these tools facilitate preparatory-to-research queries and building collaborations.
Table 2.
Data and analysis platforms available for different types of queries through the CRDC
| Type of query | Diagnose patients? | Explore a cohort? | Analyze all data? Create a new cohort? | Build collaborations? | ||
|---|---|---|---|---|---|---|
| Analysis platforms | Family-based analysis with Gregor (GeneDx DP), Emedgene, CFA, BCH Seqr | Cohort-level analysis with CFA, SKAT (GeneDx DP) | Variant summary information via BCH Aggregator | Gene phenotype associations via BCH Aggregator | Find patients with specific variants/genes with CFA, BCH Aggregator | Share variants and phenotypes internally and externally with CFA, BCH Aggregator, MME (BCH Seqr) |
| Broadly sharable research data (12,677 individuals) | Broadly available | Broadly available | Broadly available | Broadly available | Broadly available | Broadly available |
| Restricted sharing research data (8813 individuals) | Available only to contributing researcher | Available only to contributing researcher | Broadly available, aggregated onlya | Broadly available, aggregated onlya | Via honest brokerb | Available to contributing researcher and via honest brokerb |
| Non-research clinical data (9686 individuals) | Available only to referring clinician | Not available | Broadly available, aggregated onlya | Broadly available, aggregated onlya | Via honest brokerb | Not available |
DP discovery platform, CFA cohort family analysis, BCH Boston Children’s Hospital, SKAT sequence kernel association test, MME matchmaker exchange.
aOnly aggregated counts are available broadly; it is not possible to assign a specific variant to a specific individual unlike for broadly sharable data, which does so via de-identified IDs.
bThe CRDC implementation team can connect the querying researcher with the contributing researcher or referring clinician for follow-up and collaboration.
Many disease group researchers reported multiple and diverse collaborations. Internal collaborations have been integral for patient recruitment as many of the patients fall into multiple disease categories and some of the disease groups have unique combinations of phenotypes resulting in new connections between departments in the institution. 624 out of 9797 patients consented to research (6%) are enrolled in multiple research studies. Data can also be shared externally including through Genomic Information Commons28. Additionally, through the CRDC, BCH has also been established as a Matchmaker Exchange (MME)29 node with the ability to submit data integrated into one of the commonly used analysis tools, an institution-specific instance of the Broad’s Seqr23.
The CRDC has also been a critical component of the success of a recent international collaboration between four leading pediatric hospitals to investigate the diagnostic and clinical utility of rapid trio GS in infantile epilepsy30. This study recently published the results of the first 100 infants enrolled (43% diagnostic rate), 34 (34%) of whom were enrolled from BCH and supported by the CRDC. In addition to sequencing support, the BCH arm of the collaboration was able to rely on the established workflows and infrastructure to quickly get the study running. Of the published 34 cases, 44% had diagnostic findings, and this study is ongoing with 91 families enrolled at BCH (Fig. 4). The results from this rapid sequencing study have driven changes in standards of care such as offering genomic testing to patients with infantile epilepsy.
Improving clinical care
The genomics analysis platform developed by the CRDC drives a research-to-clinical loop where research genomic sequencing can have an immediate impact on clinical care (Fig. 5) because of the built-in framework to clinically confirm findings discovered by researchers and the ability for clinically generated sequencing to be easily re-analyzed or reflexed to research for further investigation. Primary variant analysis for research sequencing was performed by the research group that enrolled the participants and varied depending on the disease, researcher and analysis platform. Generally, filtering was done for population frequency, functional impact and family inheritance where applicable. Some groups used a gene list to help prioritize variants but analysis generally went beyond strict filtering on known disease genes to facilitate novel discoveries. Various annotations and filters are available via the GeneDx Discovery Platform and BCH Seqr, and AI-powered variant prioritization is available via Emedgene. Putative causative variants were then clinically confirmed via validation and classification by the CLIA-certified testing facility GeneDx and returned via the referring clinician.
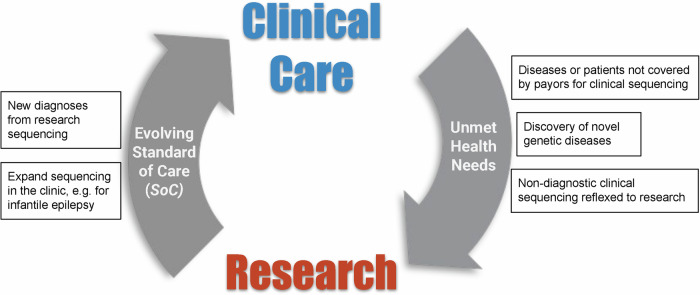
Fig. 5. The research-to-clinical loop.
The research-to-clinical loop involves taking advantage of the benefits and flexibility of research studies to close the gap on unmet health needs and then evolve the standard of care by bringing the results back to the clinic. This figure was created in Microsoft PowerPoint.
As of August 2023, 1165 patients enrolled in a CRDC research study (35% of 3353 cases analyzed at that point) had a genetic finding of interest including variants of uncertain significance (VUS) and variants in candidate disease genes, which were slated for follow-up functional analysis, and variants that were clinically confirmed (pathogenic, likely pathogenic or VUS) and returned to the patient’s health record (514 cases, 15% of cases analyzed). Crucially, the ability to clinically confirm research findings is made widely available as the samples are stored in a CLIA environment and the cost of the confirmatory testing by the sequencing lab (GeneDx) is covered by the CRDC.
Alongside research sequencing, clinical sequencing is also available at our center with 4032 patients having been tested from late 2019 through February 2024 (almost all ES with GS only becoming available very recently). The uptake of clinical exomes for patients seen in the clinic increased from 24 per month in 2016–2017 to 90 per month in 2021–2023, mirroring the increase in research genomic sequencing. Since 2019, these sequencing data have also been made available for analysis in the genomics platform, supporting re-analysis by clinicians. Additionally, storing the data in a centralized database makes it available for deeper research investigations if the individuals are enrolled in a research study (1324 of patients with clinical ES are also enrolled in a research study).
To further explore the benefit of accessible research genomic sequencing, a deeper review was performed for four cohorts: epilepsy31 (522 patients), hearing loss32 (218), cerebral palsy33 (175) and peripheral vestibular disorders (32). For all groups, a fraction of enrolled patients had had non-diagnostic previous genetic testing: 40% of the cerebral palsy cohort (mix of CMA and ES), 31% of the hearing loss cohort (mostly panel and single gene tests), 26% for peripheral vestibular disorders (single gene, panel, and ES) and 19% for epilepsy (clinical panel and/or CMA). These cases were included in the studies as the opportunity to perform ES or GS presented an improvement over the previously available tests, often limited to gene panels or CMA.
Another feature of these cohorts was that many of the patients included had phenotypes not classically offered genomic testing such as ES/GS, and for whom insurance coverage for such testing is not routine. In the epilepsy cohort, 73% of patients had an epilepsy diagnosis other than developmental and epileptic encephalopathies (DEE), and had a diagnostic rate of 14% (compared to 32% for DEE). In hearing loss (HL), 45% of patients had unilateral or asymmetric bilateral HL, which historically have not been tested genetically. In this study, 20% of those patients had diagnostic results (compared to 40% of patients with symmetric bilateral HL). Similarly, for cerebral palsy (CP), 48% had non-cryptogenic CP (patients with known acquired risk factors for CP) and a diagnostic rate of 10% compared to the 42% that had cryptogenic CP (with no known risk factors) and a diagnostic rate of 39%. And for the vestibular disorders cohort, where the contribution of Mendelian variants is largely unknown, there have been few variants clinically confirmed and returned so far, but there are a number of ongoing investigations for this gene discovery-focused study.
Discussion
Genomic sequencing has been established as a critical tool in the diagnosis of pediatric rare diseases. In this study, we described the results of five years of the CRDC, including the enrollment and sequencing of 4653 families under a broad-use research consent and from 41 different rare disease cohorts. 35% of the analyzed cases had findings of interest with 15% clinically confirmed and returned to the family. The rate of clinically confirmed findings is on the low end of published numbers for rare diseases because of ascertainment bias including enrolling patients with non-diagnostic genetic test results and presentations with less well-established genetic etiologies. Even cohorts of the same disease can have different diagnostic rates due to the underlying severity and previous investigations. As a quaternary referring center, we often see particularly complex cases that are being evaluated via several different approaches. Generally groups are not returning secondary findings, although the possibility to do so is included in the standardized consent form. Depending on the disease and research group, variant analysis can be quite broad and secondary findings are not explicitly excluded, allowing for identification if the phenotypes overlap with the primary indication. The logistics of classifying and returning secondary findings from research sequencing continues to be an area under development.
The collaborative has expanded greatly since our first report in 202011, successfully integrating the research and clinical domains, allowing for research genomic findings to have immediate clinical impact. Research groups reported that clinically-confirmed diagnoses resulted in a change in clinical care in up to 25% of cases including changes in treatment, surveillance, change in prognosis and enrollment in a clinical trial. Further, as expected, investigators reported that a precise genetic diagnosis often clarified reproductive risk and allowed families to connect with disease-specific communities for support and information. The availability of research genomics also broadened the cohort of patients who can access genomic testing, including those with diseases for which a Mendelian basis is still under evaluation. Thus, research genomic sequencing improves access to testing for families not historically referred for clinical genomic testing, sharing the benefits of genomic precision medicine with a larger community. While the availability of institution-supported research genomic testing helps address one of the barriers to more equitable access to sequencing across populations, many more obstacles need to be overcome as is described in a recent publication that reviewed racial and ethnic representation in both clinical and research genomic testing performed at BCH24.
The CRDC was established as an internally funded program as part of the institution’s research strategic plan and the successes to date indicate a path to sustainability. One example is the cost savings from alignment and standardization of genetics sequencing programs, which greatly reduces data duplication and allows for taking advantage of technologies at scale. Additionally, the data and infrastructure supplied by the CRDC escalates incoming grant funding providing a return on investment. Finally, the CRDC and the work of its associated investigators has made BCH a more specialized and effective rare disease center that, among other benefits, is opening new pathways to innovation in the therapeutic space.
As diagnostic rates increase and sequencing costs fall, hospitals are considering scaling up sequencing efforts and associated cost implications increase from hundreds of samples to thousands or tens of thousands of samples. Choosing between ES and GS then becomes a complex calculation. If we estimate GS costs approximately twice as much as ES, then GS would need to provide twice the number of genetic findings as ES to be financially efficient, which has not yet been demonstrated. One caveat to that statement is that a negative ES test often comes with additional costs as other orthogonal testing may be ordered. Additionally, there are other considerations when choosing ES or GS beyond financial cost and diagnostic rate, and these will vary considerably between patient cohorts. GS can result in increased success particularly in discovering pathogenic variants that are amenable to current precision medicine therapeutics that are being developed, for example, anti-sense oligonucleotides that target deep intronic splice variants only identifiable with GS34. In addition, there may be some presentations with a higher probability of being caused by variants that can only be found with GS such as repeat expansion diseases. However, broadly offering GS can limit the access of some patients to the benefits of sequencing as there is generally going to be a cap on the funding available, either from payers and what they are willing to cover or from research and institutional grants. Using the higher-cost option reduces the total number of patients that can receive any form of genomic sequencing, and this is likely to particularly affect those patient groups that are already less likely to have access. Ultimately, establishing a system for disease-specific decision-making processes for choosing ES or GS would help influence policy with insurance/payers.
A major impact of the CRDC has been the acceleration in the use of genomics in different departments of the hospital. This was done by supporting early investigators who may otherwise have had difficulties setting up cohorts and collaborations, which lowers the barrier of access to these research opportunities, improves career development and results in widespread skill improvements of the workforce in genomics. For instance, CRDC lead investigators include, in addition to senior investigators with independent research laboratories, junior to mid-level physician-scientist faculty as well as expert clinicians. For over half of these faculty, participation in the CRDC represents their first major study involving genomics. Our approach to data analysis involved centralized data processing and storage and distributed variant analysis workflows, which provided a higher baseline level of data processing and analysis for research cohorts that may not have their own resources but requires centralized resources to implement. Investigators still have to supply the workforce required for consenting, genetic counseling and result delivery, however, which remains a limitation.
Having a centralized processing platform allowed for switching to an updated reference genome and rolling out new variant calling methods in a systematic and comprehensive manner. The established infrastructure allowed for the rapid initiation of a pediatric severe COVID-19 cohort in the early days of the pandemic, facilitating urgent high-impact research. It has also enabled the swift rollout of a rapid-turnaround hybrid clinical/research trio GS study for infants with epilepsy, part of the International Precision Child Health Partnership (IPCHiP)30. The data repository combined with including broad research sharing in the consent forms also facilitated collaboration with ~5% of participants enrolled in multiple CRDC-aligned studies.
The availability of the clinical sequencing data has inspired a push for developing a workflow for systematic re-analysis at the institutional level. This would include routine review of all cases, automated where possible, in order to discover newly reported variants, variants in newly reported genes, and newly relevant variants/genes due to the evolving phenotypes of these pediatric patients. This is facilitated by the fact that the genomic and phenotypic data for five years of patients receiving clinical genomic sequencing can be found in the centralized platforms supported by the CRDC. Also required is a workflow for communicating variants of interest to the referring clinicians and providing access to the data. There is ongoing work to develop these workflows in an accessible and efficient manner, using CRDC-supported platforms such as BCH’s instance of Seqr.
The CRDC is well-poised to continue to adapt new technologies and methods to improve the process of diagnosing and treating pediatric rare diseases. In addition to expanding the experimental methods supported (e.g. long-read sequencing and RNA-seq), the collaborative will continue to work to expand access to these tools by further streamlining enrollment and sample collection. Incorporating new methods such as machine learning algorithms for genomic analysis and patient selection is a potential future path, as is building a bridge from diagnosis to clinical care and novel therapeutics. Investigators continue to leverage the rich phenotypic and genotypic datasets available through this collaborative to improve pediatric outcomes through research-informed healthcare.
Methods
Boston Children’s Hospital’s CRDC began on October 1, 2018 and is ongoing. The initial study design and many of the core methods have been previously published in a manuscript documenting the Pilot phase and Phase I of the collaborative11. The present report includes Phase II, which ran from October 1, 2019 through February 28, 2024. Participant enrollment, sample collection, sequencing, and data analysis were performed as previously described with a few modifications. The Boston Children’s Hospital Institutional Review Board approved all research related to this study, which complied with all relevant ethical regulations including the Declaration of Helsinki, and informed consent was obtained from all research participants and/or their legal guardians. Study data were collected and managed using REDCap electronic data capture tools hosted at BCH25,26. In March 2022, the CRDC began supporting GS in addition to ES. Similar to ES, GS was performed by GeneDx (Gaithersburg, MD), a CLIA-certified testing facility; while sequencing was conducted on a research basis, additional DNA was stored for possible clinical confirmation. DNA library preparation was performed with Illumina DNA PCR-Free Prep Tagmentation and followed by 2 × 150 paired-end sequencing on the Illumina NovaSeq 6000 platform to an average depth of coverage of at least 40X.
The centralized genomic data analysis platform was also updated in Phase II. Starting in May 2022, all genomic data were aligned to reference genome build GRCh38 with the DRAGEN (v3.9) secondary analysis platform from Illumina35. This included re-aligning all historical data sets. Identification of the following variant types was performed in Phase II: single nucleotide variants and small insertions/deletions (SNV/indels; DRAGEN), CNVs (DRAGEN), SVs (DRAGEN), disease-associated short tandem repeat expansions (STRs; Expansion Hunter36 via DRAGEN), mitochondrial genome variants (Mutect237) and mobile element insertions (MEIs; xTEA38). Variants were made available to researchers through commercial and in-house analysis platforms.
Acknowledgements
A.J.M. was supported by the NIH (1K08DK125768-01A1), American Society of Nephrology (Norman Siegel Research Scholar Career Grant 81542), Manton Center for Orphan Disease Research (Junior Faculty Award), and Boston Children’s Hospital OFD/BTREC/CTREC Faculty Career Development Fellowship.
Author contributions
C.E.F., S. Rockowitz., P.S. performed analysis and wrote the manuscript. W.G.S. and A. Sharma. performed analysis and generated figures. A.H.B., P.M.B., C.A.B., M.C., J.C., A.M.D., R.N.D., D.E.F., R.D.G., C.J., M.K., T.L., J.A.M., A.J.M., N.M., S.U.M., A.P., A.G.R., A.E.R., S. Roberts., M.G.S., D.D.S., E.S., A. Shimamura., S.B.S., S.S., J.R.T., M.C.W., M.H.W. generated and analyzed patient data and reviewed/edited the manuscript. N.C.A., W.K.C., M.I. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Data availability
The data are available internally to all BCH researchers and clinicians through the genomics analysis platform. To facilitate external access, the CRDC data has also been made available to the Genomic Information Commons project (https://www.genomicinformationcommons.org/). Code available upon request.
Competing interests
N.C.A. sits on the Boards of Directors of Novartis, Charles River Laboratories and Maze Therapeutics, and is on the Scientific Advisory Board of Dyne Therapeutics. A.J.M. is a scientific consultant for Judo Bio. M.H.W. has consulted for Illumina and Sanofi and receives speaking honoraria from Illumina and GeneDx.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turro, E. et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature583, 96–102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Splinter, K. et al. Effect of genetic diagnosis on patients with previously undiagnosed disease. N. Engl. J. Med.379, 2131–2139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright, C. F. et al. Genomic diagnosis of rare pediatric disease in the United Kingdom and Ireland. N. Engl. J. Med.388, 1559–1571 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smedley, D. et al. 100,000 genomes pilot on rare-disease diagnosis in health care—preliminary report. N. Engl. J. Med.385, 1868–1880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French, C. E. et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensiv. Care Med.45, 627–636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung, C. C. Y. et al. Rapid whole-exome sequencing facilitates precision medicine in paediatric rare disease patients and reduces healthcare costs. Lancet Reg. Heal. West. Pac.1, 100001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunke, S. et al. Australian Genomics Health Alliance Acute Care Flagship. Feasibility of ultra-rapid exome sequencing in critically Ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA323, 2503–2511 (2020). [DOI] [PMC free article] [PubMed]
- 8.Dimmock, D. et al. Project Baby Bear: rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am. J. Hum. Genet.108, 1231–1238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas, S. et al. A centralized approach for practicing genomic medicine. Pediatrics145, e20190855 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark, Z. et al. Australian genomics: outcomes of a 5-year national program to accelerate the integration of genomics in healthcare. Am. J. Hum. Genet.110, 419–426 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockowitz, S. et al. Children’s rare disease cohorts: an integrative research and clinical genomics initiative. npj Genom. Med.5, 29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippa, N. et al. Diagnostic sequencing to support genetically stratified medicine in a tertiary care setting. Genet. Med.24, 862–869 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Cohen, A. S. A. et al. Genomic answers for children: dynamic analyses of >1000 pediatric rare disease genomes. Genet. Med.24, 1336–1348 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Stranneheim, H. et al. Integration of whole genome sequencing into a healthcare setting: high diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Med.13, 40 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsmore, S. F. et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in Ill infants. Am. J. Hum. Genet.105, 719–733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark, M. M. et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. npj Genom. Med.3, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertoli-Avella, A. M. et al. Successful application of genome sequencing in a diagnostic setting: 1007 index cases from a clinically heterogeneous cohort. Eur. J. Hum. Genet.29, 141–153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfares, A. et al. Whole-genome sequencing offers additional but limited clinical utility compared with reanalysis of whole-exome sequencing. Genet. Med.20, 1328–1333 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, E. S. et al. Approaches to long-read sequencing in a clinical setting to improve diagnostic rate. Sci. Rep.12, 16945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloney, T. et al. Lessons learnt from multifaceted diagnostic approaches to the first 150 families in Victoria’s Undiagnosed Diseases Program. J. Méd. Genet.59, 748–758 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunke, S. et al. Integrated multi-omics for rapid rare disease diagnosis on a national scale. Nat. Med.29, 1681–1691 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcik, M. H. et al. Beyond the exome: what’s next in diagnostic testing for Mendelian conditions. Am. J. Hum. Genet.110, 1229–1248 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pais, L. S. et al. seqr: a web‐based analysis and collaboration tool for rare disease genomics. Hum. Mutat.43, 698–707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazier, Z. J. et al. Toward representative genomic research: the children’s rare disease cohorts experience. Ther. Adv. Rare Dis.4, 26330040231181410 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform.42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform.95, 103208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estrella, E. et al. Mendelian disorders in an interstitial cystitis/bladder pain syndrome cohort. Adv. Genet.4, 2200013 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandl, K. D. et al. The Genomics Research and Innovation Network: creating an interoperable, federated, genomics learning system. Genet. Med.22, 371–380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippakis, A. A. et al. The matchmaker exchange: a platform for rare disease gene discovery. Hum. Mutat.36, 915–921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Gama, A. M. et al. Evaluation of the feasibility, diagnostic yield, and clinical utility of rapid genome sequencing in infantile epilepsy (Gene-STEPS): an international, multicentre, pilot cohort study. Lancet Neurol.22, 812–825 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Koh, H. Y. et al. Utility of exome sequencing for diagnosis in unexplained pediatric-onset epilepsy. JAMA Netw. Open6, e2324380 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry, J. et al. Exome sequencing expands the genetic diagnostic spectrum for pediatric hearing loss. Laryngoscope133, 2417–2424 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Chopra, M. et al. Mendelian etiologies identified with whole exome sequencing in cerebral palsy. Ann. Clin. Transl. Neurol.9, 193–205 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, J. et al. A framework for individualized splice-switching oligonucleotide therapy. Nature619, 828–836 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behera, S. et al. Comprehensive and accurate genome analysis at scale using DRAGEN accelerated algorithms. bioRxiv10.1101/2024.01.02.573821 (2024).
- 36.Dolzhenko, E. et al. ExpansionHunter: a sequence-graph based tool to analyze variation in short tandem repeat regions. Bioinformatics35, 4754–4756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laricchia, K. M. et al. Mitochondrial DNA variation across 56,434 individuals in gnomAD. Genome Res. 32, gr.276013.121 (2022). [DOI] [PMC free article] [PubMed]
- 38.Chu, C. et al. Comprehensive identification of transposable element insertions using multiple sequencing technologies. Nat. Commun.12, 3836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickham, H. Ggplot2: elegant graphics for data analysis. (Springer-Verlag New York, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available internally to all BCH researchers and clinicians through the genomics analysis platform. To facilitate external access, the CRDC data has also been made available to the Genomic Information Commons project (https://www.genomicinformationcommons.org/). Code available upon request.