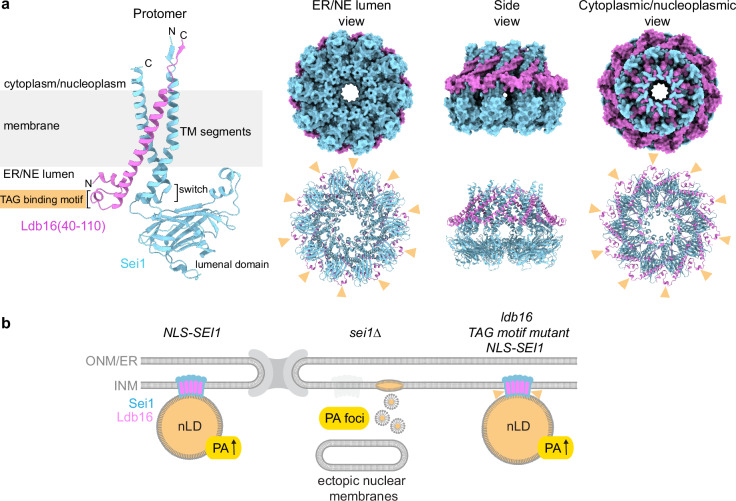
Fig. 7. Sei1 positions the TAG-binding domain of Ldb16 outside of the decameric ring.
a Cartoon representation of AlphaFold 3 model of S. cerevisiae Sei1·Ldb16(40-110) protomer (left), and both surface (right top) and cartoon (right bottom) representations of AlphaFold 3 model of Sei1·Ldb16(40-110) 10:10 ring assembly. The models are coloured by chain. Yellow arrowheads indicate putative serine/threonine-rich TAG binding motifs of Ldb16. For Sei1·Ldb16 prediction and confidence scores, see Supplementary Fig. 7. b A model illustrating the role of the Sei1-Ldb16 complex in regulating nLDs and INM lipid composition. When Sei1 is localized to the INM, nLDs with PA-enriched surfaces are formed. In the absence of Sei1, abnormal PA-rich but TAG-deficient droplets arise, accompanied by diverse defects in nuclear membrane structure. Mutations in the TAG-binding domain of Ldb16 (yellow arrowheads) reduce nLD numbers but slightly enlarge nLDs, suggesting impaired nLD biogenesis.