Abstract
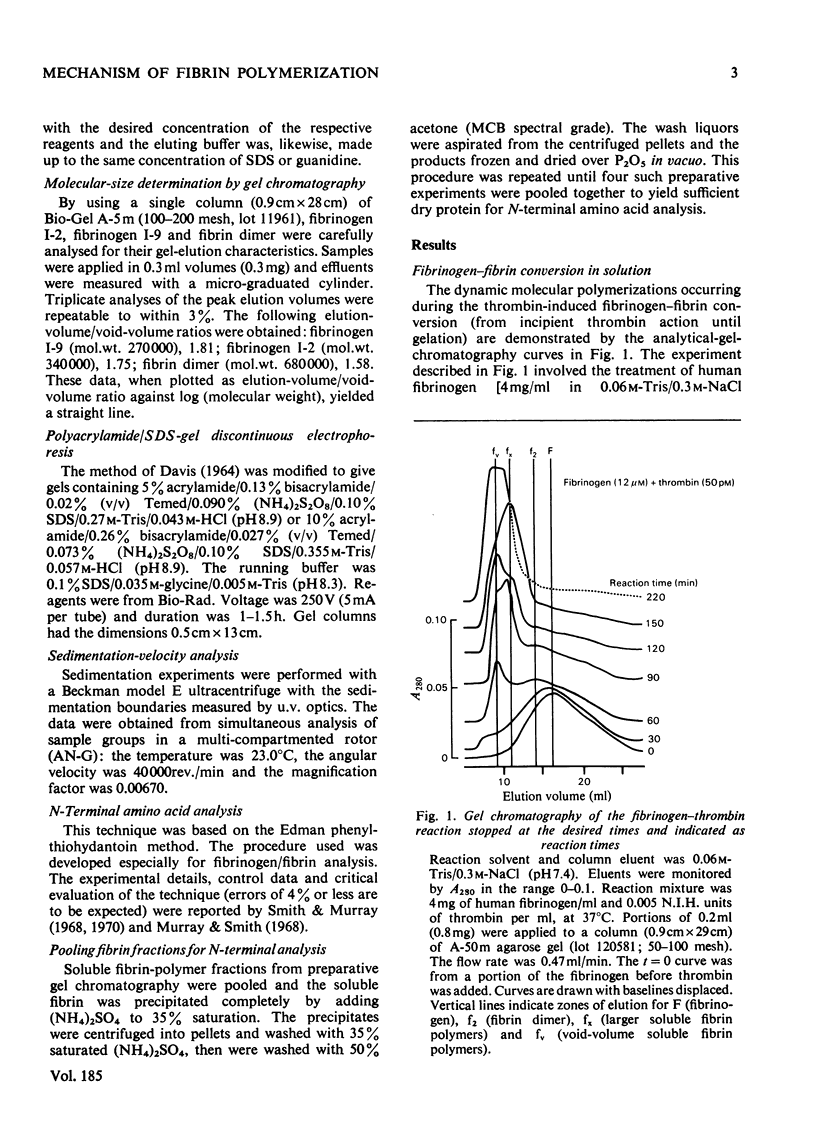
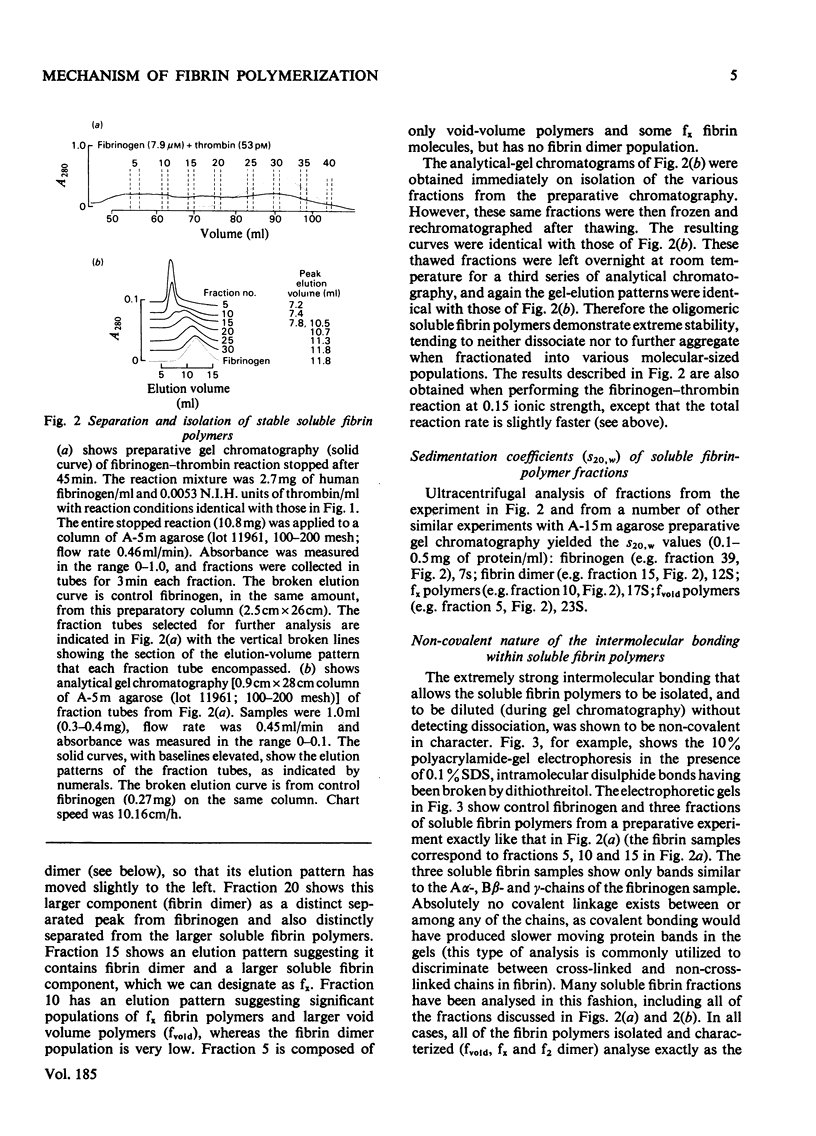
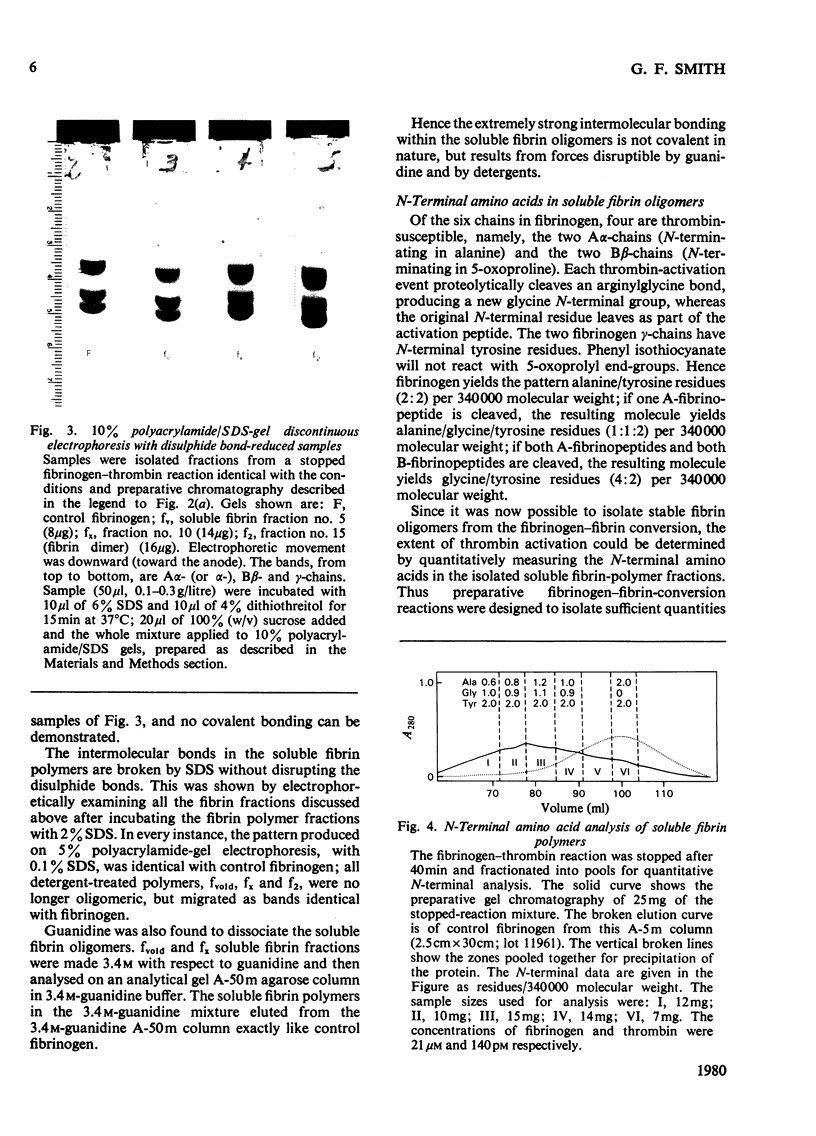
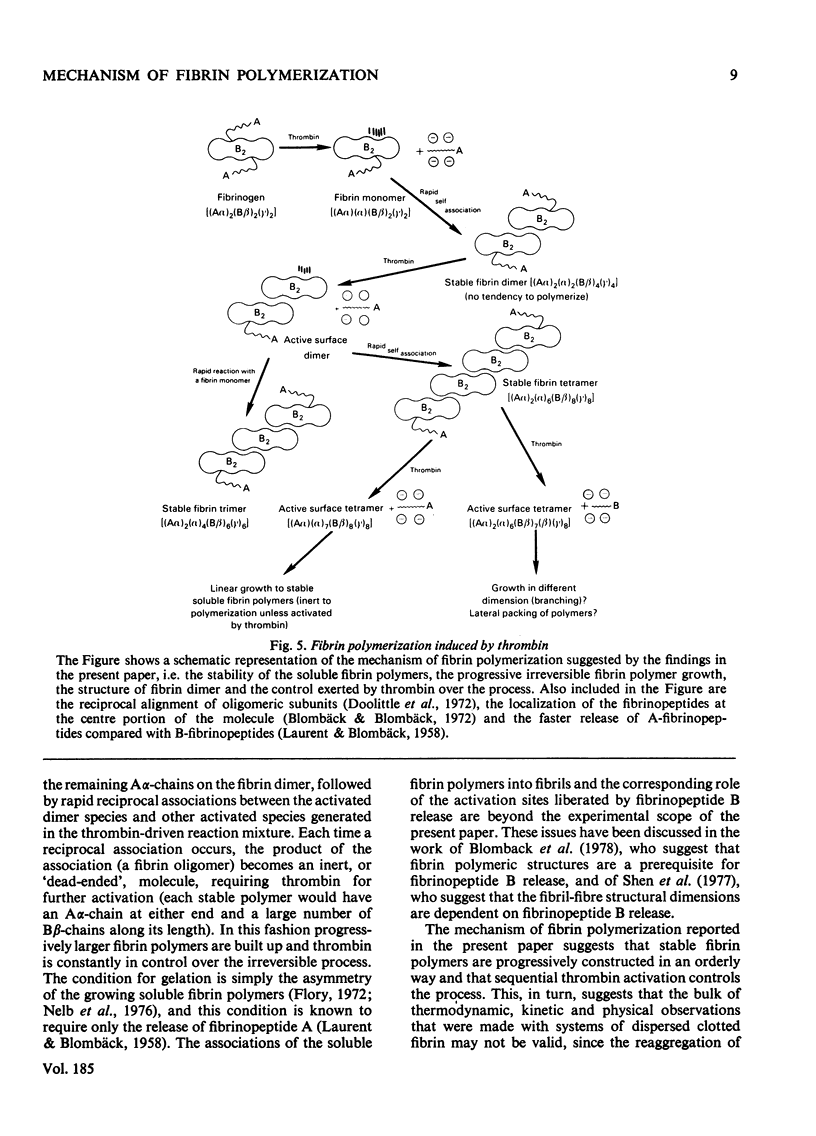
The fibrin polymers formed in solution during the earliest phase of the fibrinogen–fibrin conversion are shown to be stable soluble molecules at pH7.4 and 0.15m- or 0.3m-NaCl. The various sequential soluble fibrin polymers produced from the fibrinogen–thrombin reaction can be observed by gel chromatography and can be isolated for characterization. The mechanism of fibrin polymerization proposed from the present studies suggests that the initial event is the thrombin activation at only one of the Aα-chains in fibrinogen. The resulting highly reactive intermediate is the true fibrin monomer and it rapidly, and irreversibly, self-associates to form the stable fibrin dimer (s20.w=12S). Fibrin dimer possesses the N-terminal pattern alanine/glycine/tyrosine (1:1:2) per 340000 molecular weight, and possesses the chain structure [(α)Aα)(Bβ)2(γ)2]2. The fibrin dimer is a soluble inert molecule, but additional thrombin activation of its remaining intact Aα-chains leads to new associations into larger inert soluble fibrin polymers. In this manner progressively larger fibrin oligomers are constructed with thrombin continually in control of the process because of the necessity to repeatedly re-activate the various fibrin polymers in solution. The inert character of the soluble fibrin polymers can be explained by the reciprocal alignment of the associating molecules, which mutually consumes their active surfaces and leaves an intact Aα-chain at either end of each fibrin oligomer. The soluble fibrin polymers will proceed to further association only if thrombin activates these remaining Aα-chains, otherwise the fibrin molecules are stable indefinitely. The intermolecular associations within the soluble fibrin polymers are essentially irreversible under these nearly physiological conditions. However, the bonding is not covalent. This mechanism accounts for the clinical observations of stable fibrinogen-derived polymers in the plasma from patients undergoing thrombotic processes. Since it is shown that the intermediate fibrin polymers, themselves, are stable soluble molecules, it is no longer necessary, nor warranted, to invoke hypothetical `fibrinogen–fibrin complexes' to explain observations of fibrin solubility.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkjaersig N., Fletcher A., Burstein R. Association between oral contraceptive use and thromboembolism: a new approach to itsinvestigation based on plasma fibrinogen chromatography. Am J Obstet Gynecol. 1975 May;122(2):199–211. doi: 10.1016/s0002-9378(16)33492-5. [DOI] [PubMed] [Google Scholar]
- BETTELHEIM F. R. The clotting of fibrinogen. II. Fractionation of peptide material liberated. Biochim Biophys Acta. 1956 Jan;19(1):121–130. doi: 10.1016/0006-3002(56)90393-6. [DOI] [PubMed] [Google Scholar]
- Benabid Y., Concord E., Suscillon M. Soluble fibrin complexes: separation as a function of pH and characterization. Thromb Haemost. 1977 Feb 28;37(1):144–153. [PubMed] [Google Scholar]
- Blombäck B., Blombäck M. The molecular structure of fibrinogen. Ann N Y Acad Sci. 1972 Dec 8;202:77–97. doi: 10.1111/j.1749-6632.1972.tb16323.x. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Donati M. B., Verhaeghe R., Culasso D. E., Vermylen J. Molecular size distribution of fibrinogen derivatives formed in vitro and in vivo: a chromatographic study. Thromb Haemost. 1976 Aug 31;36(1):14–26. [PubMed] [Google Scholar]
- Doolittle R. F., Cassman K. G., Chen R., Sharp J. J., Wooding G. L. Correlation of the mode of fibrin polymerization with the pattern of cross-linking. Ann N Y Acad Sci. 1972 Dec 8;202:114–126. doi: 10.1111/j.1749-6632.1972.tb16325.x. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Edgar W., McKillop C., Howie P. W., Prentice C. R. Composition of soluble fibrin complexes in pre-eclampsia. Thromb Res. 1977 Apr;10(4):567–574. doi: 10.1016/0049-3848(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Alkjaersig N. K., O'Brien J. R., Tulevski V. Fibrinogen catabolism in the surgically treated patient and in those with postoperative venous thrombosis. Correlation of plasma fibrinogen chromatographic findings with 125I-labeled fibrinogen scan findings. J Lab Clin Med. 1977 Jun;89(6):1349–1364. [PubMed] [Google Scholar]
- Flory P. J. Molecular configuration and states of aggregation of biopolymers. Ciba Found Symp. 1972;7:109–124. doi: 10.1002/9780470719909.ch7. [DOI] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Kierulf P., Abildgaard U. Studies on soluble fibrin in plasma. I. N-terminal analysis of a modified fraction I (Cohn) from normal and thrombin-incubated plasma. Scand J Clin Lab Invest. 1971 Oct;28(2):231–240. doi: 10.3109/00365517109086905. [DOI] [PubMed] [Google Scholar]
- Kierulf P., Godal H. C. Fibrinaemia and multiple thrombi in pancreatic carcinoma. A case studied with quantitative N-terminal analysis. Scand J Haematol. 1972;9(4):370–376. doi: 10.1111/j.1600-0609.1972.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Ly B., Jakobsen E. Some aspects of the bonds interlinking soluble fibrin complexes in human plasma. Thromb Res. 1975 Jan;6(1):65–73. doi: 10.1016/0049-3848(75)90151-6. [DOI] [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Sherry S. The preparation and properties of human fibrinogen of relatively high solubility. Biochemistry. 1966 Sep;5(9):2829–2835. doi: 10.1021/bi00873a008. [DOI] [PubMed] [Google Scholar]
- Murray M., Smith G. F. Infrared spectra of microgram amounts of amino acid phenylthiohydantoins eluted from thin layers of silica gel. Anal Chem. 1968 Feb;40(2):440–442. doi: 10.1021/ac60258a055. [DOI] [PubMed] [Google Scholar]
- Nelb G. W., Gerth C., Ferry J. D. Rheology of fibrin clots. III. Shear creep and creep recovery of fine ligated and coarse unligated closts. Biophys Chem. 1976 Sep;5(3):377–387. doi: 10.1016/0301-4622(76)80050-6. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Silverberg S. A., Jesty J. Self-damping mechanism in blood coagulation. Thromb Diath Haemorrh. 1974 Sep 30;32(1):57–64. [PubMed] [Google Scholar]
- RASMUSSEN P. S. Purification of thrombin by chromatography. Biochim Biophys Acta. 1955 Jan;16(1):157–158. doi: 10.1016/0006-3002(55)90194-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., Beeler D. L., Rosenberg R. D. Activation of human prothrombin by highly purified human factors V and X-a in presence of human antithrombin. J Biol Chem. 1975 Mar 10;250(5):1607–1617. [PubMed] [Google Scholar]
- SHAINOFF J. R., PAGE I. H. Significance of cryoprofibrin in fibrinogen-fibrin conversion. J Exp Med. 1962 Nov 1;116:687–707. doi: 10.1084/jem.116.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERRY S., TROLL W., GLUECK H. Thrombin as a proteolytic enzyme. Physiol Rev. 1954 Oct;34(4):736–741. doi: 10.1152/physrev.1954.34.4.736. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Hermans J., McDonagh J., McDonagh R. P. Role of fibrinopeptide B release: comparison of fibrins produced by thrombin and Ancrod. Am J Physiol. 1977 Jun;232(6):H629–H633. doi: 10.1152/ajpheart.1977.232.6.H629. [DOI] [PubMed] [Google Scholar]
- Sherman L. A. Catabolism of fibrinogen and its derivatives. Thromb Haemost. 1977 Dec 15;38(4):809–822. [PubMed] [Google Scholar]
- Smith G. F., Bang N. U. Formation of soluble fibrin polymers. Fibrinogen degradation fragments D and E fail to form soluble complexes with fibrin monomer. Biochemistry. 1972 Aug 1;11(16):2958–2966. doi: 10.1021/bi00766a006. [DOI] [PubMed] [Google Scholar]
- Smith G. F., Craft T. J. Heparin reacts stoichiometrically with thrombin during thrombin inhibition in human plasma. Biochem Biophys Res Commun. 1976 Aug 9;71(3):738–745. doi: 10.1016/0006-291x(76)90893-7. [DOI] [PubMed] [Google Scholar]
- Smith G. F., Murray M. Direct spectrophotometric quantitation of phenylthiohydantoin derivatives of amino acids from thin layers of silica gel. Anal Biochem. 1968 May;23(2):183–195. doi: 10.1016/0003-2697(68)90350-3. [DOI] [PubMed] [Google Scholar]
- Smith G. F., Murray M. Thrombosis and blood coagulation. A study by quantitative N-terminal amino acid analysis. Arch Pathol. 1970 Mar;89(3):235–246. [PubMed] [Google Scholar]
- Smith G. F. The heparin-thrombin complex in the mechanism of thrombin inactivation by heparin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):111–117. doi: 10.1016/s0006-291x(77)80171-x. [DOI] [PubMed] [Google Scholar]
- Wessler S. Current dilemmas in the clinical use of heparin. Fed Proc. 1977 Jan;36(1):66–69. [PubMed] [Google Scholar]