Abstract
Acid sphingomyelinase deficiency (ASMD) is a rare progressive genetic disorder caused by pathogenic variants in the SMPD1 gene causing low or absent activity of the enzyme acid sphingomyelinase, resulting in subsequent accumulation of its substrate, sphingomyelin. Signs and symptoms of excessive lysosomal sphingomyelin storage, such as hepatosplenomegaly and pulmonary impairment, and in a subset of patients, progressive neurological manifestations, have long been recognized as hallmarks of the disease. Uncontrolled accumulation of sphingomyelin has important and complex downstream metabolic and immunologic consequences that contribute to the disease burden. This review article expounds on the complex and multifaceted role of sphingomyelin in the pathophysiology of ASMD and discusses the animal studies and human interventional trials demonstrating that sphingomyelin and its related metabolites are linked to ASMD clinical manifestations, disease burden, and response to treatment. The relationship between the diverse manifestations of ASMD and sphingomyelin accumulation and the connections between sphingomyelin clearance and reversal of the noncentral nervous system manifestations by olipudase alfa therapy also are described.
Keywords: Acid sphingomyelinase deficiency type A/B, Acid sphingomyelinase deficiency type B, Chronic acid sphingomyelinase deficiency, Lyso-sphingomyelin, Lysosomal storage disorder
Introduction
Acid sphingomyelinase deficiency (ASMD) is a rare lysosomal storage disease caused by pathogenic variants in the SMPD1 gene, resulting in deficient activity of the lysosomal enzyme acid sphingomyelinase (ASM) and the resultant intracellular accumulation of its substrate, sphingomyelin.1 ASMD encompasses a broad spectrum of disease with variable age of onset, symptoms at presentation, and degree/type of organ involvement. Historically known as type A and B Niemann-Pick disease, it is now referred to as ASMD and is delineated into infantile neurovisceral (ASMD type A, a rapidly progressive and invariably fatal neurodegenerative disease), chronic visceral (ASMD type B, a more slowly progressive form without known neurologic involvement), and chronic neurovisceral (ASMD type A/B, an intermediate phenotype between A and B with more slowly progressive neurologic manifestations) forms.2, 3, 4
ASMD is inherited in an autosomal recessive manner, and more than 200 pathogenic or likely pathogenic SMPD1 variants have been identified to date, (https://www.ncbi.nlm.nih.gov/clinvar) some with established genotype-phenotype relationships.5 The overall estimated incidence of ASMD is 0.4 to 0.6 in 100,000 newborns, with higher prevalence in specific populations.6 ASMD is underrecognized with missed and delayed diagnoses; therefore, current estimates likely underrepresent the actual prevalence.2
The most severe form of ASMD (ASMD type A, neurovisceral phenotype) is a fatal neurodegenerative disease of infancy with death usually by 2 to 3 years of age. It is characterized by rapidly progressive psychomotor degeneration, failure to thrive, hepatosplenomegaly, and cherry-red macula.7 In contrast, ASMD type B8 and ASMD type A/B9 (chronic visceral and chronic neurovisceral phenotypes, respectively) are characterized by later onset of hepatosplenomegaly that may be accompanied by interstitial pulmonary disease and fibrosis, elevated liver function tests, dyslipidemia, and in advanced cases, by liver fibrosis or cirrhosis (Table 12,3,8,10, 11, 12, 13, 14). Clinical consequences may include spontaneous or trauma-related spleen rupture, respiratory impairment, potentially fatal pulmonary infections, anemia and leukopenia, delayed bone development, growth, and puberty; osteopenia with increased fracture risk; and progressive liver fibrosis leading to hepatic dysfunction or liver failure. Long-standing hypercholesterolemia may increase the risk of coronary artery disease. In addition to visceral disease, patients with ASMD type A/B have neurologic involvement that can include neurocognitive delay, hypotonia, ataxia, and peripheral neuropathy.8,9 Chronic ASMD phenotypes are associated with early mortality in both children and adults.8,15, 16, 17 Until recently, clinical management was limited to addressing individual symptoms to minimize complications and morbidity arising from progressive disease.18 The first disease-specific treatment for ASMD, olipudase alfa (recombinant human ASM), was approved in 2022 for the non-central nervous system (CNS) manifestations of ASMD in children and adults and has been shown to reverse signs and symptoms of sphingomyelin storage and associated clinical disease manifestations.19
Table 1.
Disease manifestations and burden of illness from natural history studies in children and adults with acid sphingomyelinase deficiency
| Tissue | Disease Manifestation | Frequency in ASMD Type B or A/B (Natural History Studies) | Characterization |
|---|---|---|---|
| Liver | Hepatomegaly | ∼90%2,3 | Common presenting symptom; cause of abdominal distension and early satiety; values can be up to 4× normal; liver fibrosis/cirrhosis10 |
| Abnormal LFTs | ∼50%3 | Elevated ALT and AST and total bilirubin | |
| Dyslipidemia | ∼90%3,11 | Atherogenic profile: low HDL with high LDL, high total cholesterol, and high triglycerides; may contribute to increased risk of cardiovascular events | |
| Lung | ILD as measured by high-resolution computed tomography | ∼90%2,3 | Caused by sphingomyelin-laden alveolar macrophages that impair gas exchange12 Respiratory infections are common; pneumonia is a frequent cause of death |
| % Predicted DLCO <80% | ∼70%8 | Worsens over time in natural history studies10 | |
| Abnormal FVC, FEV1, TLC | ∼50%10 | Usually indicative of restrictive (rather than obstructive) pulmonary disease | |
| Dyspnea | 81%3 | Associated with new/worsening ILD, decreased exercise tolerance | |
| Spleen | Splenomegaly/hypersplenism | ∼90%3 | Common presenting symptom; cause of abdominal distension and early satiety; values can be >27 multiples of normal. Risk of spleen rupture |
| Skeletal | Growth deficits (below CDC 50th percentile for age) | ∼90%3 | The characteristic pattern in ASMD patients who are symptomatic in early childhood is growth restriction associated with delayed skeletal maturation. Not all patients with chronic ASMD have short stature in adulthood. |
| Delayed bone maturation/puberty | Most adolescents10 | Foamy macrophages in bone marrow and sea blue histiocytes are similar to what is seen in Gaucher disease and indistinguishable from NPC and GM1 gangliosidosis and other lysosomal diseases13 | |
| Bone pain | 39%10 | Can be debilitating and impact quality of life and activitiesof daily living | |
| Low BMD, osteopenia/ osteoporosis by WHO classification | Most adults14 | BMD is low in children and adults; pathologic fractures can occur in patients with advanced disease | |
| Brain | Hypotonia, ataxia, cognitive decline | Variable | Most prominent in type A/B patients; minimal or absent in type B patients |
| Biomarker | Elevated plasma lyso-sphingomyelin | 100% | Deacylated form of sphingomyelin measurable in plasma. Mean 40× upper limit of normal in ASMD patients enrolled in clinical trials |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASMD, acid sphingomyelinase deficiency; BMD, bone mineral density; CDC, Centers for Disease Control and Prevention; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HDL, high-density lipoprotein; ILD, interstitial lung disease; LDL, low-density lipoprotein; LFTs, liver function tests; NPC, Niemann-Pick disease type C; TLC, total lung capacity; WHO, World Health Organization.
In this article, we review the role of sphingomyelin in the pathophysiology of ASMD and explore how olipudase alfa treatment can alleviate ASMD disease burden in children and adults with ASMD.
Lysosomal function and the role of acid sphingomyelinase
For many years, the biology and function of lysosomes were defined solely by their catabolic function. Thus, the pathophysiology of lysosomal storage disorders, including ASMD, was assumed to be the direct consequence of defective degradation and disposal of accumulated substrates. It is now recognized that the autophagic-lysosomal pathway is involved in many cellular functions, including the regulation of multiple signaling pathways and the adaptation to environmental stimuli.20 The expanded awareness of lysosome function has substantially affected our understanding of lysosomal storage disorder pathophysiology, and it is now clear that substrate accumulation often triggers complex pathogenic cascades that are responsible for disease pathology.21
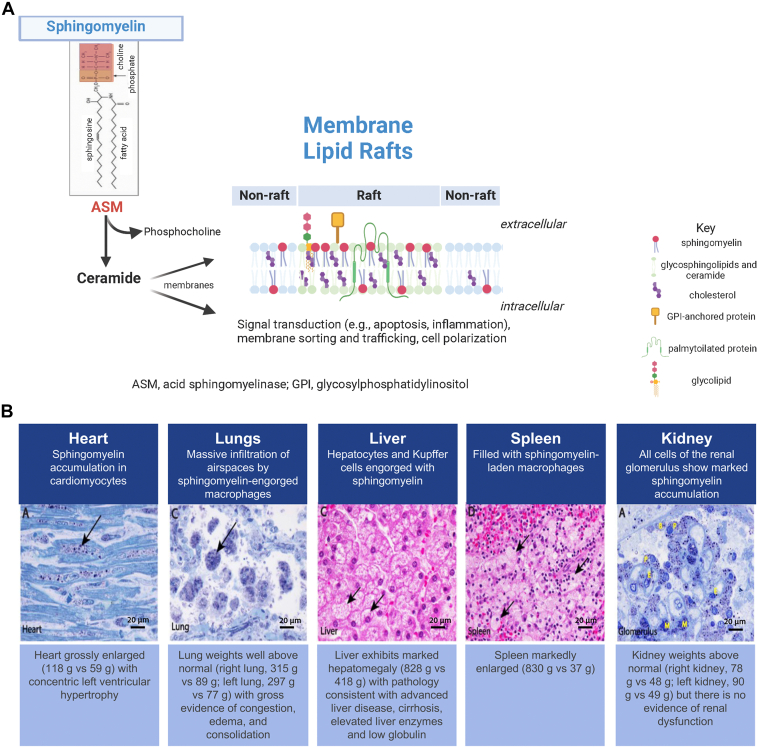
ASM is 1 of 5 sphingomyelinases encoded by distinct genes in humans that vary in characteristics, including subcellular location and optimal pH activity, with ASM primarily being found in lysosomes and cell membranes.22 Sphingomyelin is the most prevalent sphingolipid in cells and makes up approximately a third of the total lipids in cell membranes.23 Sphingomyelin is hydrolyzed by ASM to form ceramide and phosphocholine, with resulting downstream effects on membrane structure, signal transduction pathways, and the sphingolipid metabolism (Figure 1A).24,25 ASM is unique among lysosomal enzymes in having functions outside the lysosome, including translocation to the plasma membrane under stress conditions where it initiates signaling through the breakdown of sphingomyelin to ceramide.26 Sphingomyelin and ceramide are major component of lipid microdomains, or “raft” structures in the plasma membrane, which are highly enriched in membrane proteins and are key sites of cell signaling (Figure 1A24). Signaling is dependent upon the fluidity of the membrane rafts, which is controlled in part by the ratio of sphingomyelin and cholesterol to ceramide and other sphingolipids.27 Significant changes in the sphingomyelin content of membrane rafts within plasma membranes contribute to the development of many disorders and disease states, including insulin resistance, fatty liver disease, obesity, and inflammation.28
Figure 1.
Sphingomyelin catabolism by acid sphingomyelinase (ASM) (A) and sphingomyelin accumulation in ASM deficiency (ASMD) (B). A. Sphingomyelin is hydrolyzed by ASM to form ceramide and phosphocholine with resulting downstream effects on signal transduction pathways and membrane structure driven in part by the ratio of sphingomyelin and cholesterol to ceramide and other sphingolipids in membrane microdomains called lipid rafts, which accumulate specific types of proteins (created in part using BioRender.com). B. Widespread sphingomyelin accumulation in tissues and organs in ASMD. Histopathology of postmortem tissue samples from a child with ASMD type A. Other organs with accumulation of sphingomyelin included adrenals, ganglion cells, pancreas, bone marrow, intestine, brain (cortex and brainstem), tongue, and esophagus. Scale bar = 20 microns (images reproduced from Thurberg et al24 under CC BY-NC-ND license http://creativecommons.org/licenses/BY-NC-ND/4.0/).
In ASMD, reduced or impaired ASM activity leads to the accumulation of sphingomyelin in lysosomes and cells of the monocyte/macrophage lineage that reside in reticuloendothelial tissues, with appearance of lipid-laden macrophage and parenchymal cells (called foam cells)29 in multiple tissues and organs (Figure 1B).24 Because of the normal recycling of the lysosomes to the cell surface, as well as the role of these organelles in autophagy, over time, the accumulated lysosomal sphingomyelin is distributed to other cell compartments, leading to broad cellular dysfunction. Liver, spleen, lung, and bone marrow are among the earliest and most prominent organs and tissues affected by sphingomyelin accumulation,1 but accumulation occurs in multiple cell types and organs, including the adrenal cortex, heart, kidney, lymph nodes, and neurons (in neurologically impaired individuals).2,24
Because it is not always feasible or advisable to biopsy target organs to determine sphingomyelin levels, a plasma biomarker that reflects tissue sphingomyelin levels is advantageous. Lyso-sphingomyelin (lyso-SM) is the deacylated form of sphingomyelin and accumulates when sphingomyelin degradation is impaired. Lyso-SM is likely produced from sphingomyelin in lysosomes via the enzyme acid ceramidase, which has a broad deacylase activity toward several sphingolipids. Because lyso-SM is less lipophilic compared with sphingomyelin, it is more likely than sphingomyelin to be released from membranes and into the circulation. Consequently, plasma lyso-SM levels are significantly elevated in individuals with ASMD.30, 31, 32, 33 Plasma lyso-SM is a highly specific biomarker of ASMD,34,35 and the levels correlate with ASMD disease burden.30,36 In addition, lyso-SM accumulates in skin lesions of individuals with atopic dermatitis and may be related to disruption of the skin barrier. The enzyme responsible for the production of lyso-SM in atopic dermatitis is sphingomyelin deacylase, which is identical to the beta subunit of acid ceramidase.37 It is not known if lyso-SM accumulates in the skin of ASMD patients and how this may affect skin barrier function.
In clinical trials of enzyme replacement therapy (ERT) for ASMD, the mean elevation of lyso-SM at trial baseline was more than 40 times the upper limit of normal, and changes in plasma lyso-SM have been used to assess both initial and long-term treatment responses to ERT31, 32, 33,38, 39, 40, 41 as described in detail below in the sections on ASMD pathogenesis in children and adults.
The role of sphingomyelin accumulation in ASMD pathogenesis: Animal models of ASMD
The effects of sphingomyelin accumulation in tissues and organs affected by ASMD have been extensively studied using a mouse model, the ASM knockout mouse (ASMKO). ASMKO mice develop progressive sphingomyelin accumulation with disease manifestations similar to those occurring in humans.42 Animals appear normal at birth, but by 2 months of age exhibit significant accumulation of sphingomyelin in liver, spleen, heart, lungs, and brain compared with wild-type mice, with a concomitant decrease in lifespan secondary to neurologic disease and weight loss.
Sphingomyelin content is up to 6-fold higher in the brains of ASMKO mice compared with age-matched wild-type mice,43 and this accumulation correlates with loss of Purkinje cells, neuroinflammation (eg, astrogliosis and microglial activation), and the appearance of lysosomal lipid inclusions in neurons and other cells throughout most brain regions.42 Degeneration of cerebellar Purkinje cells begins by 7 weeks of age, with near complete degeneration by 20 weeks. Performance of ASMKO mice in rotarod tests, an assay for coordination and muscle strength, was similar to controls at 5 to 7 weeks of age but sharply deteriorated between 7 and 20 weeks of age.44 Age-progressive functional deficits, including ataxia and memory loss, are also correlated with sphingomyelin accumulation, and death occurs between 24 and 36 weeks secondary to the neurologic disease.
In addition to sphingomyelin, other sphingolipids, including gangliosides, ceramides, and the downstream product of ceramide hydrolysis, sphingosine, are increased in the brains of ASMKO mice.43 This complex pattern of sphingolipid accumulation in different brain cell types and within different subcellular compartments, including neuronal membranes, indicates the likelihood of multiple cell signaling abnormalities and/or disordered macromolecule trafficking in ASMD. For example, changes in sphingomyelin and other raft lipids affect the expression, distribution, and function of endocannabinoid receptors, which are integral membrane proteins within lipid raft structures of neuronal membranes.45 Abnormal expression of the endocannabinoid receptor CB1 has been demonstrated in neurons of ASMKO mice and in an individual with ASMD type A, leading to reduced endocannabinoid signaling.46 Addition of sphingomyelin to normal neurons recreated this endocannabinoid signaling abnormality, which was then corrected by treatments that reduce sphingomyelin.46
Thus, although the full impact of elevated sphingomyelin on cell function continues to be elucidated, these findings demonstrate that the clinical manifestations in ASMD result from complex interactions initiated by sphingomyelin storage but ultimately caused by diverse lipid, inflammatory, and other cellular abnormalities.
Sphingomyelin accumulation also affects pulmonary function. Lung physiology and function in ASMKO mice are affected by sphingomyelin accumulation similar to lung pathology seen in human ASMD, with the exception that lung fibrosis does not occur in the mice, likely because of the shortened animal lifespan.47,48 Many pulmonary cellular abnormalities are found but the alveolar macrophage is the most significantly affected cell type in both ASMKO mice and in humans with ASMD. Airspaces and bronchoalveolar lavage fluid of ASMKO mice contain significantly more macrophages and neutrophils than age-matched wild-type mice.48 By 10 weeks of age, the macrophages in ASMKO mice exhibit foamy cytoplasm and small/multiple nuclei in contrast to macrophages of wild-type mice and have significantly higher amounts of sphingomyelin.48 Macrophages from bronchoalveolar lavage fluid of ASMKO mice exhibit impaired function, including disrupted surfactant metabolism,49 generation of reactive oxidants,48 and phagocytosis.48, 49, 50 Sphingomyelin accumulation in the ASMKO macrophages correlates with increased inflammatory cell recruitment and production of proinflammatory cytokines in the airspaces and lavage fluid.48,50 These altered inflammatory responses in combination with abnormalities in surfactant processing due to elevated levels of sphingomyelin likely contribute to impaired host defense mechanisms after infection.51 Notably, studies of cystic fibrosis (CF) in mice and humans have further revealed the important role of ASM in pulmonary biology and host defense. When healthy individuals are exposed to pulmonary pathogens, including P aeruginosa and S aureus, ASM activity in pulmonary epithelial cells is elevated and is required to produce ceramide and the downstream lipid, sphingosine, resulting in the activation of cell signaling pathways to resolve inflammation and infection.52 In CF mice and cell lines from individuals with CF, the ASM signaling pathways are disrupted, resulting in an overwhelming IL-8 cytokine response, decreased bacterial uptake, and reduced apoptotic responses,53 leading to chronic inflammation and infection that can be corrected by modulating ASM activity. Thus, metabolism of sphingomyelin by ASM in pulmonary cell membranes plays a critical role in host defense to various pathogens and could be disrupted in ASMD patients.
Progressive accumulation of sphingomyelin is also found in liver macrophages (Kupffer cells) and hepatocytes of ASMKO mice,42 and recapitulates the pathophysiology seen in humans with ASMD.3,16 Increased sphingomyelin leads to elevated cathepsin B in ASMKO mouse hepatic stellate cells, which is associated with increases in markers of liver fibrosis, including α-smooth muscle actin, transforming growth factor-β, and pro-collagen-α1, and increased liver fibrosis in response to damage with carbon tetrachloride.54 Age-related liver fibrosis is occasionally seen in the ASMKO mice, but as with lung fibrosis, it is likely limited because of the shortened lifespan of these animals. As with other organs, tissue damage in the liver of ASM-deficient cells and patients is likely due to a complex interaction of cell-type-specific lipid accumulation, leading to cell dysfunction and death, as well as chronic inflammation, leading to the release of toxic inflammatory mediators into the tissue matrix.
In addition to these findings, studies in ASMKO mice and individuals with ASMD have shown that sphingomyelin accumulation leads to defective CD1-mediated antigen presentation and decreased numbers of invariant natural killer T cells.55 Replacement of ASM activity corrected these defects, demonstrating the tight link between cellular sphingomyelin metabolism and immunity.
Exogenous human ASM, whether administered as a purified protein via ERT or expressed via gene therapy, reduces sphingomyelin buildup in target tissues in a dose-dependent manner in the ASMKO mice.56, 57, 58, 59, 60, 61 For example, intravenous dosing of recombinant human ASM every other day for 14 days led to dose-dependent reductions of sphingomyelin in liver, spleen, lung, and heart in ASMKO mice, and long-term administration of this enzyme once per week for 15 weeks showed additional sphingomyelin reductions in the kidney and lung.56 Single intravenous doses of recombinant human ASM developed for clinical use demonstrated similar dose-related reductions of sphingomyelin in liver, spleen, lung, and kidney.57 Intracerebroventricular infusion of recombinant ASM in the ASMKO mouse reduced lysosomal accumulation and sphingomyelin in the brain and corrected CNS manifestations.60 Sphingomyelin accumulation in tissues of ASMKO mice was also prevented by gene therapy with adenoviral and adeno-associated viral vectors encoding human ASM58,61 and by hematopoietic stem cell gene therapy using retroviral vectors encoding human ASM.59 In addition to these studies, generation of knockin transgenic mice, in which specific ASMD mutations with residual ASM activity were expressed in the ASMKO mouse, revealed that ASM activity at ∼8% of normal could completely prevent the occurrence of sphingomyelin accumulation in nonneurologic tissues and substantially slow the accumulation in the CNS62,63 (and unpublished observations). These mice survived over 1 year of age, whereas ASMKO mice generally do not survive past 6 months of age.
In summary, studies using ASMD animal models have characterized the relationship between sphingomyelin accumulation and ASMD pathophysiology and demonstrated the potential for decreased sphingomyelin accumulation to reduce disease burden, resulting in the clinical development of an ERT for the non-CNS manifestations of ASMD.
The role of sphingomyelin accumulation in ASMD pathogenesis: Assessment in children and adults with ASMD
ASMD disease burden
Table 12,3,8,10, 11, 12, 13, 14 collates the major disease manifestations and burden of illness in children and adults with ASMD from natural history studies. Splenomegaly, hepatomegaly, and pulmonary disease occur in 90% of individuals with ASMD and contribute to significant morbidity and mortality.3,15 Spleen volume in multiples of normal (MN), determined as proportion of body weight, can be increased as much as 27-fold,3 and sphingomyelin concentrations are 17 to 42 times higher than normal.64 Histology assessments show microscopic alterations of the spleen with the red pulp being replaced by foamy macrophages accompanied by a reduced amount of lymphoid tissue.65 In a longitudinal natural history study of 29 adults and children with ASMD, in which all individuals with intact spleens had splenomegaly, the incidence of abnormal leukocyte and platelet counts increased over time and was 34% and 54%, respectively, after 10 years.8 However, spleen volume does not appear to correlate with platelet count, possibly because of normal fluctuations in platelet levels and/or platelet dysfunction.10
Hepatomegaly occurs in most children and adults with ASMD (Table 1).2,3,8,10, 11, 12, 13, 14 Histopathological assessments show sphingomyelin concentrations in liver are 5 to 40 times higher versus controls64 with a common pattern of increased foamy macrophages, foamy hepatocytes, and sphingomyelin-laden Kupffer cells over time.24,66 ASMD-related hepatomegaly often presents with abnormal liver chemistries and a proatherogenic lipid profile, as well as fibrosis and cirrhosis.66, 67, 68 Liver biopsies among adults with ASMD show variable degrees of fibrosis, sphingomyelin accumulation, and macrophage infiltration.66 Liver failure is a significant cause of death regardless of age.16,69 Autopsies of individuals with ASMD show severe sinusoid packing, hepatic cord pressure, pallor, vacuolization, cirrhosis, and inflammation.24,64,65
Pulmonary dysfunction in ASMD manifests as interstitial lung disease (ILD) due to accumulation of sphingomyelin in alveolar macrophages residing in the interalveolar septae with distortion of architecture and structural damage.47,70,71 Furthermore, sphingomyelin-laden macrophages accumulate in the alveolar space, making air exchange difficult (due to an “alveolar filling defect”). Therefore, compromised lung diffusion capacity can be detected in individuals with chronic ASMD2,8,15,16,72,73 and ∼70% have decreased gas exchange as measured by a % predicted hemoglobin-adjusted diffusion capacity of the lung for carbon monoxide (DLCO) < 80% (Table 1).2,3,8,10, 11, 12, 13, 14 In a prospective natural history study, the percent-predicted DLCO decreased by 12.5% in children and 10.4% in adults over 11 years, with the most impaired function observed in patients with dyspnea and severe ILD.3 High-resolution computed tomography of the lungs of most individuals with ASMD shows marked ground-glass opacities caused by foamy macrophages74 and radiological evidence of interstitial fibrotic disease is frequently present as well.75 The infiltrative pulmonary process is slowly progressive,15,16,47,71,76 and chronic obstructive pulmonary disease and severe chronic respiratory deficiency develop in some cases.77 Pulmonary dysfunction contributes to the ASMD disease burden and severity of sequela,72 and respiratory complications are a leading cause of death in individuals with ASMD types B and A/B.3,15,16 Autopsy specimens show evidence of bronchopneumonia and pneumonitis across ASMD subtypes.24,65 For example, clinical and pathologic autopsy findings of a 3-year-old boy with ASMD type A showed massive infiltration of airspaces by sphingomyelin-engorged macrophages with a mixed population of inflammatory cells. These findings correlated with the above-normal weight of both lungs and gross evidence of congestion, edema, and consolidation and were consistent with the child’s early breathing difficulties at 17 months and subsequent clinical pneumonia.24
Correlation of tissue sphingomyelin accumulation and/or levels of plasma lyso-SM with degree of tissue damage and functional impairment in individuals with ASMD
Case studies and series using pathology data from autopsies and tissue biopsies along with biomarker analyses describe chronic tissue alterations related to progressive sphingomyelin accumulation over time, as well as correlations between baseline disease severity and the degree of ASM deficiency. In liver and spleen, the accumulated concentrations of sphingomyelin, total phospholipid, and total cholesterol are significantly higher in those with ASMD type A compared with ASMD type B.64 Similarly, plasma lyso-SM levels are elevated and positively correlate with clinical severity across ASMD subtypes: levels are higher among individuals with ASMD type A versus types B and A/B, and positively correlated with clinical severity.36 Levels of sphingomyelin in tissues and correlations with the degree of tissue damage were reported from autopsy findings in the 3-year-old male with ASMD type A referred to above.24 Profound sphingomyelin accumulation was present in virtually every organ and cell type (Figure 1B).24 These cases highlight that the extensive cellular and organ involvement observed in the most severe ASMD phenotype (type A) is a harbinger of the manifestations in the less severe ASMD type B and A/B phenotypes. Although these less severe manifestations may be more subtle, less recognized, and possibly silent, they are likely to result in irreversible changes over time.
Interventional olipudase alfa clinical trials provided a unique opportunity to investigate the relationship between tissue sphingomyelin levels, plasma lyso-SM levels, and disease burden in patients with ASMD types B and A/B. Table 2 31,32,38, 39, 40 summarizes the entry criteria and baseline disease burden for adults and children enrolled in the olipudase alfa clinical trials for which regulatory approvals were based: a phase 1b trial in adults and long-term extension trial,33,38,40,68,78 an open-label pediatric trial (ASCEND-Peds) and long-term extension trial,32,39 and a placebo-controlled trial in adults (ASCEND) with open-label extension.31,41
Table 2.
Trial entry criteria and baseline clinical values in 3 olipudase alfa clinical trials of previously untreated children and adults with ASMD
|
Clinical Trials of Olipudase Alfa Enzyme Replacement Therapy | |||
|---|---|---|---|
| Phase1b,40 and Long-Term Trial38 NCT01722526 NCT02004704 |
ASCEND31 NCT02004691 |
ASCEND-Peds32 and Long-Term Trial39 NCT02292654 NCT02004704 |
|
| Trial design | Open-label | Placebo-controlled (1:1 randomization) | Open-label |
| Participants | 5 adults | 36 adults | 20 children |
| Age range, y | 22 to 47 | 18.6 to 65.9 | 1.5 to 17.5 |
| Key inclusion criteria |
|
|
|
| Baseline Values | Normal Ranges | |||
|---|---|---|---|---|
| Elevation of plasma lyso-sphingomyelin | 40 × ULN | 45 × ULN | 63 × ULN | ULN 9.99 μg/mL |
| % Liver tissue area occupied by sphingomyelin mean ± SD min, max |
33.3% ± 17.8 (n = 5) 9.8, 53.8 |
29.8% ± 9.8 (n = 35) 10.8, 58.6 |
Liver biopsies were not done in children | 0 |
| Spleen volume MN mean ± SD min, max |
12.8 ± 4.8 (n = 5) 7.4, 17.9 |
11.5 ± 4.5 (n = 35) 4.9, 20.9 |
19.0 ± 4.8 (N = 20) 7.8, 36.4 |
NA |
| Liver volume MN mean ± SD min, max |
1.74 ± 0.48 (n = 5) 1.21, 2.21 |
1.52 ± 0.44 (n = 35) 0.83, 3.11 |
2.65 ± 0.74 (N = 20) 1.69, 4.19 |
NA |
| % Predicted DLCO adjusted for Hgba Mean ± SD min, max |
53.2% ± 17.6 (n = 5) 39.5, 77.1 |
49.6% ± 10.9 (n = 35) 25.4, 70.1 |
54.8% ± 14.2 (n = 9)a 27.0, 71.6 |
≥75% or 80% |
| high-resolution computed tomography ground glass appearance scoreb mean ± SD min, max |
0.99 ± 0.95 (n = 5) 0.13, 2.33 |
0.68 ± 0.72 (n = 35) 0.0, 3.0 |
0.79 ± 0.75 (n = 20) 0.0, 3.0 |
0 |
| Alanine amino transferase (U/L) mean ± SD |
41.8 ± 32.3 (n = 5) | 41 ± 27.5 (n = 35) | 63.0 ± 32.2 (n = 20) | 8-37 U/L (0.13-0.62 ukat/L) |
| Aspartate aminotransferase (U/L) mean ± SD |
41.4 ± 30.9 (n = 5) | 43.7 ± 31.0 (n = 35) | 84.1 ± 52.2 (n = 20) | 10-34 U/L (0.17-0.57 ukat/L) |
| HDL cholesterol (mg/dL) mean ± SD |
19.5 ± 10.6 (n = 5) | 22.0 ± 8.2 (n = 35) | 16.9 ± 6.1 (n = 20) | >40 mg/dL (>1.04 mmol/L) optimal ≥ 60 mg/dL (>1.55 mmol/L) |
| LDL cholesterol (mg/dL) mean ± SD |
109.9 ± 18.8 (n = 5) | 140.0 ± 32.4 (n = 35) | 150.6 ± 63.1 (n = 19) | <129 mg/dL (<3.34 mmol/L) optimal < 100 mg/dL (<2.59 mmol/L) |
| Triglycerides (mg/dL) mean ± SD |
187.9 ± 115.5 (n = 5) | 186.0 ± 76.9 (n = 35) | 199.0 ± 93.1 (n = 20) | <150 mg/dL (<1.69 mmol/L) |
Conventional to SI conversions: for alanine amino transferase and aspartate aminotransferase multiply U/L by 0.0167; for HDL and LDL cholesterol multiply mg/dL by 0.0259; for triglycerides, multiply mg/dL by 0.0113.
DLCO, pulmonary diffusing capacity of carbon monoxide; MN, multiples of normal; NA, not applicable; ULN, upper limit of normal.
Only patients ≥ 5 years could perform the DLCO test.
0 = no disease; 1 = mild [1%-25% lung volume affected]; 2 = moderate [26%-50%]; 3 = severe [51%-100%].
Correlation of sphingomyelin and lyso-sphingomyelin levels with clinical assessments in olipudase alfa trials
Levels of sphingomyelin in liver and/or plasma lyso-SM levels at trial baseline correlated with ASMD clinical characteristics and disease burden. In liver tissue of individuals with ASMD, 10% to 60% (mean: ∼30%) of tissue area was shown to be occupied by sphingomyelin (Table 2 31,38, 39, 40). Liver sphingomyelin levels correlated with liver volume MN (r = 0.48, P = .0017) but not with liver transaminase levels or plasma lipoprotein levels.79 Plasma lyso-SM levels were elevated between 12 to 144 times above normal (upper limit of normal 9.9 μg/L) at baseline in adults and children with ASMD (Table 2 31,32,38, 39, 40), and levels correlated positively with liver volume (adults: r = 0.673; P < .0001; children: r = 0.734; P = .0002) and alanine aminotransferase (adults: r = 0.630; P < .0001; children: r = 0.518; P = .0192), and negatively with high-density lipoprotein (HDL) cholesterol (adults: r = −0.554; P = .0002; children: r = −0.536; P = .014).30
Greater than 90% of adults and children in the phase 1b, ASCEND and ASCEND-Peds olipudase alfa clinical trials had radiologic evidence of ILD with increased ground-glass opacities caused by infiltration of foamy, sphingomyelin-filled macrophages (Figure 2B) (Table 2).31,32,38, 39, 40, 41,78 Percent-predicted DLCO was abnormal in all individuals (as dictated by study entry criterion), whereas mild deficits in pulmonary function tests, such as forced vital capacity, were indicative of restrictive lung disease.31,38,39 No lung tissue sphingomyelin data are available from olipudase alfa clinical trials; however, baseline plasma lyso-SM levels inversely correlated with baseline DLCO in adults and children (adults: r = −0.436; P = .0049; children: r = −0.700; P = .0358).30 Spleen volumes were required to be ≥6 MN in adults and ≥5 MN in children for olipudase alfa clinical trial eligibility. Spleen biopsies were not performed because of the risk of splenic rupture; therefore, sphingomyelin levels in splenic tissue are not available. Baseline plasma lyso-SM levels correlated positively with spleen volume in adults but for unknown reasons, not in children (adults: r = 0.808; P < .0001; children: r = 0.215; P = .3626).30
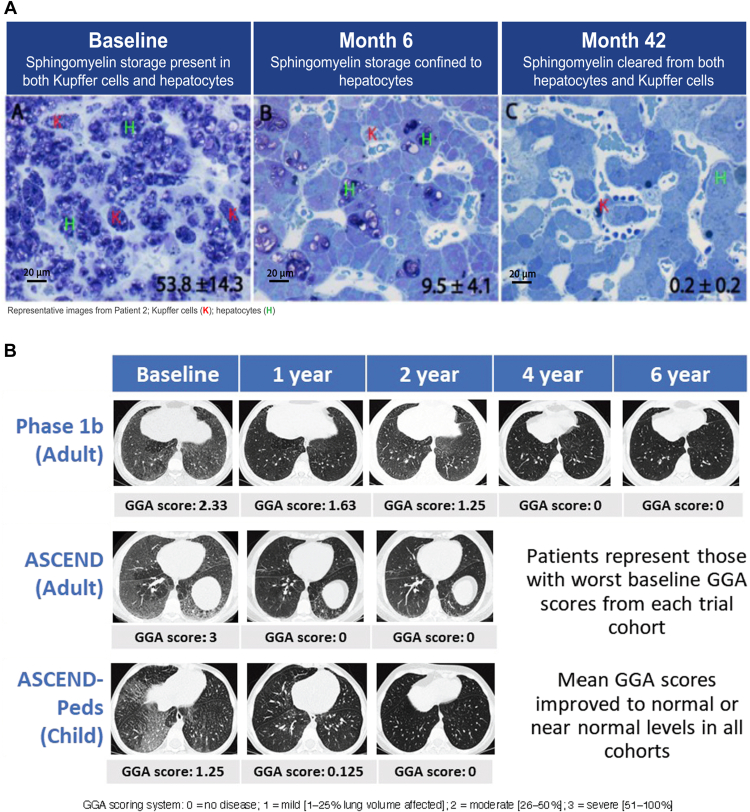
Figure 2.
Sphingomyelin accumulation in liver and lungs of individuals with ASMD before and after olipudase alfa treatment. A. Sphingomyelin liver burden at baseline and after olipudase alfa treatment in an adult with ASMD. Modified toluidine blue stain highlights sphingomyelin in dark purple at baseline, month 6, and month 42. Sphingomyelin was cleared from Kupffer cells (K) and reduced in hepatocytes (H). Percent tissue area occupied by sphingomyelin (as calculated by MetaMorph analysis) ± standard deviation, shown in the lower right corners. Scale bar = 20 microns (images reproduced from Thurberg et al78 under CC BY-NC-ND license http://creativecommons.org/licenses/BY-NC-ND/4.0/). B. Ground-glass appearance in lung at baseline and after olipudase alfa treatment (high-resolution computed tomography lung imaging). Illustrative samples from adult and pediatric clinical trials. Ground-glass appearance scores are shown below images (images reproduced from Lachmann et al,38 Wasserstein et al,41 and Diaz et al39 under CC BY-NC-ND license http://creativecommons.org/licenses/BY-NC-ND/4.0/).
Olipudase alfa treatment reduces sphingomyelin accumulation in target tissues in children and adults with ASMD
ERT with olipudase alfa supplements deficient ASM activity, allowing accumulated sphingomyelin to be broken down into ceramide and phosphocholine in a dose-dependent manner. Histological analysis of liver biopsy data in adults treated with olipudase alfa showed substantial clearance of sphingomyelin in Kupffer cells and hepatocytes with a mean percent change from baseline in the area occupied by sphingomyelin in liver of −86.6% after 6 months and −99.7% after 3 years68,78 (Figure 2A). Similar results were observed in a placebo-controlled trial in adults with ASMD in which the mean percent change from baseline in the area occupied by sphingomyelin in liver was −92.7% in the olipudase alfa group versus +10.9% in the placebo group at week 52.31 No liver biopsies were performed in the clinical trial of olipudase alfa in children with ASMD; however, both children and adults had elevated plasma lyso-SM, which decreased by ∼70% within the first 6 to 9 months of olipudase alfa treatment, and plateaued with prolonged treatment (Figure 3).31, 32, 33,38,39,41
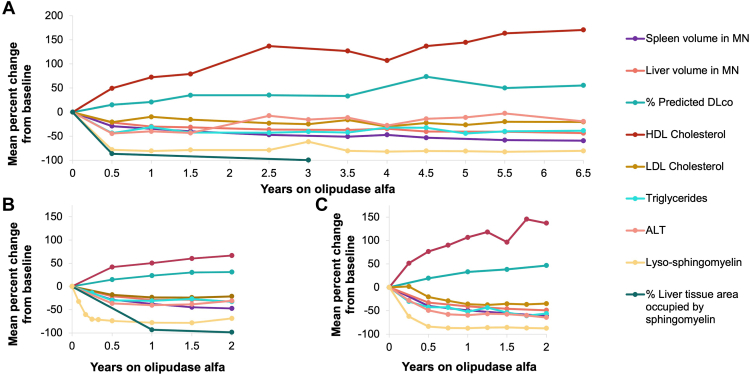
Figure 3.
Time course of improvement in key clinical endpoints after long-term olipudase alfa treatment in children and adults.38,39,41 See Table 231,32,38, 39, 40 for clinical trial eligibility requirements and baseline parameters. A. Percent change from baseline during treatment with olipudase alfa in adults enrolled in the phase 1b and continuing in the long-term trial (n = 5).38 B. Percent change from baseline during treatment with olipudase alfa in adults enrolled in the ASCEND trial and continuing in the trial extension (n = 36, the n for individual data points varies). Includes data for individuals in the placebo group after crossing over to olipudase alfa).41 C. Percent change from baseline during treatment with olipudase alfa for children enrolled in the ASCEND-Peds trial and continuing in the long-term trial (n = 20, the n for individual data points varies).39 Note that there were no liver biopsies performed in the pediatric trial; therefore, there are no data for liver sphingomyelin content.
Sphingomyelin clearance and reduced lyso-SM levels after olipudase alfa treatment occur in parallel with improvements in clinical manifestations of ASMD
Sphingomyelin clearance and decreased plasma lyso-SM levels are accompanied by reduced organomegaly, normalization of lipid profiles and liver transaminases in all individuals, improved pulmonary function, and catch-up growth and bone maturation in children. In the placebo-controlled ASCEND trial in adults, clinical improvements were seen only in the olipudase-alfa-treated group during the first year of treatment.31 Figure 338,39,41 shows treatment responses for major clinical variables as a function of time on olipudase alfa treatment graphed with the timelines for improvements in sphingomyelin tissue burden and plasma lyso-SM levels.
Across all trials, all individuals who had hepatomegaly at baseline had a reduction in liver volume with olipudase alfa treatment, with the largest decreases occurring in the first 6 to 9 months of treatment (Figure 3).31,32,38, 39, 40, 41 After 2 years, liver volumes in most patients were in the mild to no hepatomegaly range (≤1.25 MN), with further incremental decreases observed for 6.5 years in the longest running trial (Figure 3).38,39,41 Reductions in liver volume were accompanied by normalization of liver transaminase levels, reflecting improvement in liver function. At baseline, 50% of adults and 80% of children had abnormal alanine aminotransferase and aspartate transaminase (AST) values at baseline. In most individuals, values normalized in the first year of treatment and remained normal with sustained treatment.31,32,38,39,41 Across olipudase alfa trials, most individuals had a proatherogenic lipid profile characterized by low HDL cholesterol, elevated low-density lipoprotein cholesterol, total cholesterol, and triglyceride levels. HDL cholesterol levels gradually improved (increased) over time with olipudase alfa treatment, whereas low-density lipoprotein cholesterol, total cholesterol, and triglyceride levels decreased rapidly to normal or near normal levels within the first year of treatment and remained at these levels with sustained treatment.31,32,38,39,41,68,78
Trial participants had baseline spleen volumes ≥5 MN as per trial entry criterion, with individual values ranging from 5 to 36 MN. Spleen volumes decreased with olipudase alfa treatment in all children and adults over time, with the largest decreases seen in the first year (Figure 3).31, 32, 33,38,39,41 Despite the substantial and highly statistically significant spleen size reduction, spleen volume did not fully normalize in all individuals even with prolonged treatment, similar to observations with ERT or substrate reduction therapy for other lysosomal storage diseases, such as Gaucher disease, in which responses to treatment are affected by pretreatment spleen volume.80 The reason for this is not known but may be due to tissue fibrosis.
A percent-predicted DLCO of <80% was an entry criterion for the ASCEND and phase 1b adult trials, and mean baseline values were ∼50%. At baseline, in all the adult and pediatric clinical trials, there was a negative correlation between DLCO and ground-glass appearance score (the higher the ground-glass appearance score the lower the DLCO), suggesting a relationship between sphingomyelin accumulation in lung tissue and the alveolar space, and impaired gas exchange: (r values: ASCEND: −0.456, P = .0052; ASCEND-Peds: −0.661, P = .0525, phase 1b: −0.900, P = .037481 and unpublished observations). With 1 year of olipudase alfa treatment, DLCO improved 23% in ASCEND, 33% in ASCEND-Peds, and 21% in the phase 1b trial (Figure 3),31, 32, 33,38,39,41 with continued improvement over time on treatment (55% improvement by year 6.5 in phase 1b; Figure 3).38,39,41,81 Other measures of lung function, including forced vital capacity, total lung capacity, and forced expiratory volume in 1 second, were in the low-normal range at baseline in most patients and gradually improved or normalized with treatment.31, 32, 33,38,39,41,81 Lung high-resolution computed tomography imaging showed clearance of ground-glass opacities by 2 to 4 years of treatment in most patients, as well as improvements in interstitial lung disease scores (Figure 2B).38,39,41,78,81
Perspective and Conclusions
In individuals with ASMD and in ASM-deficient mice, sphingomyelin accumulates within multiple parenchymal cell types, as well as in the monocyte/macrophage cell lineage of the reticuloendothelial system, resulting in splenomegaly, hepatomegaly, ILD, and bone marrow infiltration. In a subset of patients, sphingomyelin accumulates in the CNS as well, including neurons and glial cells. Progressive, uncontrolled accumulation of sphingomyelin has important metabolic and immunologic consequences that contribute to the disease burden. As the major substrate of ASM, defining the role of sphingomyelin accumulation in ASMD is important in understanding the overall ASMD disease burden and the role of disease modifying therapies.
The role of sphingomyelin in the pathophysiology of ASMD is complex and multifaceted, and the interplay between sphingomyelin and other lipids and their downstream effects on cell signaling and function is not fully understood in ASMD. Although further research is needed, it is clear from animal studies and human interventional trials that the level of sphingomyelin and metabolites are linked to the clinical manifestations, disease burden, and response to treatment.
Studies in ASMKO mice have shown that sphingomyelin is toxic when it accumulates in tissues and that treatment strategies delivering functional ASM (eg, ERT or gene therapy) reduce sphingomyelin levels, correct abnormal histopathology, and in some cases, correct functional deficits. In individuals with ASMD, sphingomyelin accumulation results in structural changes and functional loss in various organs and tissues, and the degree of sphingomyelin accumulation correlates with the degree of tissue damage. Enzyme replacement therapy with olipudase alfa provides an exogenous source of functional ASM that clears sphingomyelin from non-CNS cells and tissues affected by ASMD. It also leads to reduction of downstream sphingomyelin metabolites, such as glycosphingolipids, ceramides, and sphingosine. In adults and children with ASMD treated with olipudase alfa, the reduction of sphingomyelin levels is associated with clinical improvement and often normalization or near normalization of tissue structure and/or organ function. Liver biopsy data and the radiological evidence of clearance of lung ground-glass opacity support the concept that improvement in symptoms and organ function in ASMD is related to clearance of sphingomyelin from tissues.
ASMD is characterized by progressive accumulation of sphingomyelin and progressive disease burden. In ASMD type A, accumulation of sphingomyelin in the CNS and other organs results in rapid deterioration in infants starting at 3 to 6 months of age and death by 3 years of age. For ASMD type B, disease burden in children and adults primarily reflects sphingomyelin accumulation in visceral organs with a later onset and slower progression, whereas in ASMD type A/B, disease burden is related to sphingomyelin accumulation in visceral organs, as well as the CNS, with a slower rate of accumulation and longer survival than type A. Recent research has identified naturally occurring sphingomyelins that are enriched in the brains and neurons of ASMKO mice compared with wild-type mice, with a dramatic increase in one form (sphingomyelin 16:0) for which plasma levels correlate with brain pathology.82 There are currently no effective treatments for the CNS manifestations in ASMD type A, but it is clear that future treatments should address sphingomyelin accumulation because this is the underlying cause of the pathophysiology in all ASMD patients. Olipudase alfa is currently the only approved treatment for the non-CNS manifestations of ASMD. Given the often clinically inconspicuous, progressive accumulation of sphingomyelin in cells and tissues of type B and A/B patients, and the prospect for future fibrosis and other irreversible organ damage, early diagnosis and initiation of treatment should be key objectives to manage the disease burden in these individuals with ASMD.
Data Availability
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents redacted to protect privacy. Details on Sanofi’s data sharing criteria for published and unpublished data and the process for requesting access can be found at: https://www.vivli.org/.
Conflict of Interest
Monica Kumar, Mario Aguiar, Kelly George, Vanessa Davidson, and Lisa Underhill are employees and stockholders of Sanofi. Beth L. Thurberg, Andreas Jessel, and Holly Wong are former employees of Sanofi. Beth L. Thurberg has received honorary and consulting fees from Sanofi. Edward H. Schuchman has received grant support, royalties, honoraria, consulting fees, and travel support from Sanofi.
Acknowledgments
Funding
Funding was provided by Sanofi, Cambridge, MA, USA. Support for medical writing and editing assistance provided by Patrice Ferriola, PhD was funded by Sanofi.
Author Contributions
Conceptualization: M.K., M.A., A.J., V.D., K.G.; Investigation and Methodology: M.K., M.A., A.J., V.D., K.G., L.U., H.W., B.L.T., E.H.S.; Writing-original draft: M.K., M.A., A.J., V.D., K.G., L.U., H.W., B.L.T., E.H.S.; Writing-review and editing subsequent drafts: M.K., M.A., A.J., V.D., K.G., L.U., H.W., B.L.T., E.H.S.
ORCID
Edward H. Schuchman: http://orcid.org/0000-0002-6638-2385
Footnotes
This article was invited and the Article Publishing Charge (APC) was waived.
References
- 1.Schuchman E.H., Desnick R.J. Types A and B Niemann-Pick disease. Mol Genet Metab. 2017;120(1-2):27–33. doi: 10.1016/j.ymgme.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGovern M.M., Avetisyan R., Sanson B.J., Lidove O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD) Orphanet J Rare Dis. 2017;12(1):41. doi: 10.1186/s13023-017-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGovern M.M., Wasserstein M.P., Bembi B., et al. Prospective study of the natural history of chronic acid sphingomyelinase deficiency in children and adults: eleven years of observation. Orphanet J Rare Dis. 2021;16(1):212. doi: 10.1186/s13023-021-01842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGovern M.M., Dionisi-Vici C., Giugliani R., et al. Consensus recommendation for a diagnostic guideline for acid sphingomyelinase deficiency. Genet Med. 2017;19(9):967–974. doi: 10.1038/gim.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasserstein M., Schuchman E. In: GeneReviews. Pagon R., Adamm M., Ardinger H., editors. University of Washington; 2023. Acid sphingomyelinase deficiency.https://www.ncbi.nlm.nih.gov/books/NBK1370/ [Google Scholar]
- 6.Kingma S.D.K., Bodamer O.A., Wijburg F.A. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract Res Clin Endocrinol Metab. 2015;29(2):145–157. doi: 10.1016/j.beem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.McGovern M.M., Aron A., Brodie S.E., Desnick R.J., Wasserstein M.P. Natural history of Type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66(2):228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 8.Wasserstein M.P., Desnick R.J., Schuchman E.H., et al. The natural history of type B Niemann-Pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114(6):e672–e677. doi: 10.1542/peds.2004-0887. [DOI] [PubMed] [Google Scholar]
- 9.Wasserstein M.P., Aron A., Brodie S.E., Simonaro C., Desnick R.J., McGovern M.M. Acid sphingomyelinase deficiency: prevalence and characterization of an intermediate phenotype of Niemann-Pick disease. J Pediatr. 2006;149(4):554–559. doi: 10.1016/j.jpeds.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 10.McGovern M.M., Wasserstein M.P., Giugliani R., et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 2008;122(2):e341–e349. doi: 10.1542/peds.2007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGovern M.M., Pohl-Worgall T., Deckelbaum R.J., et al. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145(1):77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Jones S.A., McGovern M., Lidove O., et al. Clinical relevance of endpoints in clinical trials for acid sphingomyelinase deficiency enzyme replacement therapy. Mol Genet Metab. 2020;131(1-2):116–123. doi: 10.1016/j.ymgme.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Geberhiwot T., Wasserstein M., Wanninayake S., et al. Consensus clinical management guidelines for acid sphingomyelinase deficiency (Niemann-Pick disease types A, B and A/B) Orphanet J Rare Dis. 2023;18(1):85. doi: 10.1186/s13023-023-02686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserstein M., Godbold J., McGovern M.M. Skeletal manifestations in pediatric and adult patients with Niemann Pick disease type B. J Inherit Metab Dis. 2013;36(1):123–127. doi: 10.1007/s10545-012-9503-0. [DOI] [PubMed] [Google Scholar]
- 15.McGovern M.M., Lippa N., Bagiella E., Schuchman E.H., Desnick R.J., Wasserstein M.P. Morbidity and mortality in type B Niemann-Pick disease. Genet Med. 2013;15(8):618–623. doi: 10.1038/gim.2013.4. [DOI] [PubMed] [Google Scholar]
- 16.Cassiman D., Packman S., Bembi B., et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann-Pick disease type B and B variant): literature review and report of new cases. Mol Genet Metab. 2016;118(3):206–213. doi: 10.1016/j.ymgme.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Pulikottil-Jacob R., Dehipawala S., Smith B., et al. Survival of patients with chronic acid sphingomyelinase deficiency (ASMD) in the United States: a retrospective chart review study. Mol Genet Metab Rep. 2024;38 doi: 10.1016/j.ymgmr.2023.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasserstein M., Dionisi-Vici C., Giugliani R., et al. Recommendations for clinical monitoring of patients with acid sphingomyelinase deficiency (ASMD) Mol Genet Metab. 2019;126(2):98–105. doi: 10.1016/j.ymgme.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syed Y.Y. Olipudase Alfa in non-CNS manifestations of acid sphingomyelinase deficiency: a profile of its use. Clin Drug Investig. 2023;43(5):369–377. doi: 10.1007/s40261-023-01270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballabio A., Bonifacino J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol. 2020;21(2):101–118. doi: 10.1038/s41580-019-0185-4. [DOI] [PubMed] [Google Scholar]
- 21.Parenti G., Medina D.L., Ballabio A. The rapidly evolving view of lysosomal storage diseases. EMBO Mol Med. 2021;13(2) doi: 10.15252/emmm.202012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang H., Jin S., Tan F., Xu Y., Lu Y., Wu T. Physiological functions and therapeutic applications of neutral sphingomyelinase and acid sphingomyelinase. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111610. [DOI] [PubMed] [Google Scholar]
- 23.Slotte J.P. Biological functions of sphingomyelins. Prog Lipid Res. 2013;52(4):424–437. doi: 10.1016/j.plipres.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Thurberg B.L. Autopsy pathology of infantile neurovisceral ASMD (Niemann-Pick disease type A): clinicopathologic correlations of a case report. Mol Genet Metab Rep. 2020;24 doi: 10.1016/j.ymgmr.2020.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goñi F.M. Sphingomyelin: what is it good for? Biochem Biophys Res Commun. 2022;633:23–25. doi: 10.1016/j.bbrc.2022.08.074. [DOI] [PubMed] [Google Scholar]
- 26.Smith E.L., Schuchman E.H. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008;22(10):3419–3431. doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slotte J.P. Cholesterol-sphingomyelin interactions in cells—effects on lipid metabolism. Subcell Biochem. 1997;28:277–293. [PubMed] [Google Scholar]
- 28.Chakraborty M., Jiang X.C. Sphingomyelin and its role in cellular signaling. Adv Exp Med Biol. 2013;991:1–14. doi: 10.1007/978-94-007-6331-9_1. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins R.W., Canals D., Hannun Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21(6):836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasserstein M., Giugliani R., Jones S., et al. P349: plasma lyso-sphingomyelin, biomarker for acid sphingomyelinase deficiency: correlations with baseline disease and response to olipudase alfa treatment in clinical trials. Genet Med Open. 2023;1 [Google Scholar]
- 31.Wasserstein M., Lachmann R., Hollak C., et al. A randomized, placebo-controlled clinical trial evaluating olipudase alfa enzyme replacement therapy for chronic acid sphingomyelinase deficiency (ASMD) in adults: one-year results. Genet Med. 2022;24(7):1425–1436. doi: 10.1016/j.gim.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Diaz G.A., Jones S.A., Scarpa M., et al. One-year results of a clinical trial of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency. Genet Med. 2021;23:1543–1550. doi: 10.1038/s41436-021-01156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasserstein M.P., Diaz G.A., Lachmann R.H., et al. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): safety and efficacy in adults treated for 30 months. J Inherit Metab Dis. 2018;41(5):829–838. doi: 10.1007/s10545-017-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubaski F., Burlina A., Pereira D., et al. Quantification of lysosphingomyelin and lysosphingomyelin-509 for the screening of acid sphingomyelinase deficiency. Orphanet J Rare Dis. 2022;17(1):407. doi: 10.1186/s13023-022-02560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchar L., Sikora J., Gulinello M.E., et al. Quantitation of plasmatic lysosphingomyelin and lysosphingomyelin-509 for differential screening of Niemann-Pick A/B and C diseases. Anal Biochem. 2017;525:73–77. doi: 10.1016/j.ab.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Breilyn M.S., Zhang W., Yu C., Wasserstein M.P. Plasma lyso-sphingomyelin levels are positively associated with clinical severity in acid sphingomyelinase deficiency. Mol Genet Metab Rep. 2021;28 doi: 10.1016/j.ymgmr.2021.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teranishi Y., Kuwahara H., Ueda M., et al. Sphingomyelin deacylase, the enzyme involved in the pathogenesis of atopic dermatitis, is identical to the beta-subunit of acid ceramidase. Int J Mol Sci. 2020;21(22):8789. doi: 10.3390/ijms21228789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lachmann R.H., Diaz G.A., Wasserstein M.P., et al. Olipudase Alfa Enzyme Replacement Therapy for Acid sphingomyelinase Deficiency (ASMD): sustained Improvements in Clinical Outcomes after 6.5 years of Treatment in Adults. Orphanet J Rare Dis. 2023;18(1):94. doi: 10.1186/s13023-023-02700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz G.A., Giugliani R., Guffon N., et al. Long-term safety and clinical outcomes of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency: two-year results. Orphanet J Rare Dis. 2022;17(1):437. doi: 10.1186/s13023-022-02587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserstein M.P., Jones S.A., Soran H., et al. Successful within-patient dose escalation of olipudase alfa in acid sphingomyelinase deficiency. Mol Genet Metab. 2015;116(1-2):88–97. doi: 10.1016/j.ymgme.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasserstein M.P., Lachmann R., Hollak C., et al. Continued improvement in disease manifestations of acid sphingomyelinase deficiency for adults with up to 2 years of olipudase alfa treatment: open-label extension of the ASCEND trial. Orphanet J Rare Dis. 2023;18(1):378. doi: 10.1186/s13023-023-02983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horinouchi K., Erlich S., Perl D.P., et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet. 1995;10(3):288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 43.Scandroglio F., Venkata J.K., Loberto N., et al. Lipid content of brain, brain membrane lipid domains, and neurons from acid sphingomyelinase deficient mice. J Neurochem. 2008;107(2):329–338. doi: 10.1111/j.1471-4159.2008.05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macauley S.L., Sidman R.L., Schuchman E.H., Taksir T., Stewart G.R. Neuropathology of the acid sphingomyelinase knockout mouse model of Niemann-Pick A disease including structure-function studies associated with cerebellar Purkinje cell degeneration. Exp Neurol. 2008;214(2):181–192. doi: 10.1016/j.expneurol.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Schuchman E.H., Ledesma M.D., Simonaro C.M. New paradigms for the treatment of lysosomal storage diseases: targeting the endocannabinoid system as a therapeutic strategy. Orphanet J Rare Dis. 2021;16(1):151. doi: 10.1186/s13023-021-01779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartoll A., Toledano-Zaragoza A., Casas J., Guzmán M., Schuchman E.H., Ledesma M.D. Inhibition of fatty acid amide hydrolase prevents pathology in neurovisceral acid sphingomyelinase deficiency by rescuing defective endocannabinoid signaling. EMBO Mol Med. 2020;12(11) doi: 10.15252/emmm.201911776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Ranke F.M., Pereira Freitas H.M., Mançano A.D., et al. Pulmonary involvement in Niemann-Pick disease: a state-of-the-art review. Lung. 2016;194(4):511–518. doi: 10.1007/s00408-016-9893-0. [DOI] [PubMed] [Google Scholar]
- 48.Dhami R., He X., Gordon R.E., Schuchman E.H. Analysis of the lung pathology and alveolar macrophage function in the acid sphingomyelinase—deficient mouse model of Niemann-Pick disease. Lab Invest. 2001;81(7):987–999. doi: 10.1038/labinvest.3780311. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami M., Dhami R., Schuchman E.H. Alveolar lipoproteinosis in an acid sphingomyelinase-deficient mouse model of Niemann-Pick disease. Am J Physiol Lung Cell Mol Physiol. 2003;284(3):L518–L525. doi: 10.1152/ajplung.00258.2002. [DOI] [PubMed] [Google Scholar]
- 50.Poczobutt J.M., Mikosz A.M., Poirier C., et al. Altered macrophage function associated with crystalline lung inflammation in acid sphingomyelinase deficiency. Am J Respir Cell Mol Biol. 2021;64(5):629–640. doi: 10.1165/rcmb.2020-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinto C., Sousa D., Ghilas V., Dardis A., Scarpa M., Macedo M.F. Acid sphingomyelinase deficiency: a clinical and immunological perspective. Int J Mol Sci. 2021;22(23) doi: 10.3390/ijms222312870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grassmé H., Jendrossek V., Riehle A., et al. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9(3):322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 53.Yu H., Zeidan Y.H., Wu B.X., et al. Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41(3):367–375. doi: 10.1165/rcmb.2008-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moles A., Tarrats N., Fernández-Checa J.C., Marí M. Cathepsin B overexpression due to acid sphingomyelinase ablation promotes liver fibrosis in Niemann-Pick disease. J Biol Chem. 2012;287(2):1178–1188. doi: 10.1074/jbc.M111.272393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melum E., Jiang X., Baker K.D., et al. Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin. Nat Immunol. 2019;20(12):1644–1655. doi: 10.1038/s41590-019-0504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda S.R., He X., Simonaro C.M., et al. Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14(13):1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 57.Murray J.M., Thompson A.M., Vitsky A., et al. Nonclinical safety assessment of recombinant human acid sphingomyelinase (rhASM) for the treatment of acid sphingomyelinase deficiency: the utility of animal models of disease in the toxicological evaluation of potential therapeutics. Mol Genet Metab. 2015;114(2):217–225. doi: 10.1016/j.ymgme.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Samaranch L., Pérez-Cañamás A., Soto-Huelin B., et al. Adeno-associated viral vector serotype 9-based gene therapy for Niemann-Pick disease type A. Sci Transl Med. 2019;11(506) doi: 10.1126/scitranslmed.aat3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miranda S.R., Erlich S., Friedrich V.L., Jr., Gatt S., Schuchman E.H. Hematopoietic stem cell gene therapy leads to marked visceral organ improvements and a delayed onset of neurological abnormalities in the acid sphingomyelinase deficient mouse model of Niemann-Pick disease. Gene Ther. 2000;7(20):1768–1776. doi: 10.1038/sj.gt.3301300. [DOI] [PubMed] [Google Scholar]
- 60.Dodge J.C., Clarke J., Treleaven C.M., et al. Intracerebroventricular infusion of acid sphingomyelinase corrects CNS manifestations in a mouse model of Niemann-Pick A disease. Exp Neurol. 2009;215(2):349–357. doi: 10.1016/j.expneurol.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Passini M.A., Bu J., Fidler J.A., et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci U S A. 2007;104(22):9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones I., He X., Katouzian F., Darroch P.I., Schuchman E.H. Characterization of common SMPD1 mutations causing types A and B Niemann-Pick disease and generation of mutation-specific mouse models. Mol Genet Metab. 2008;95(3):152–162. doi: 10.1016/j.ymgme.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ledesma M.D., Prinetti A., Sonnino S., Schuchman E.H. Brain pathology in Niemann Pick disease type A: insights from the acid sphingomyelinase knockout mice. J Neurochem. 2011;116(5):779–788. doi: 10.1111/j.1471-4159.2010.07034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanier M.T. Biochemical studies in Niemann-Pick disease. I. Major sphingolipids of liver and spleen. Biochim Biophys Acta. 1983;750(1):178–184. doi: 10.1016/0005-2760(83)90218-7. [DOI] [PubMed] [Google Scholar]
- 65.Crocker A.C., Farber S. Niemann-Pick disease: a review of eighteen patients. Medicine (Baltimore) 1958;37(1):1–95. doi: 10.1097/00005792-195802000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Thurberg B.L., Wasserstein M.P., Schiano T., et al. Liver and skin histopathology in adults with acid sphingomyelinase deficiency (Niemann-Pick disease type B) Am J Surg Pathol. 2012;36(8):1234–1246. doi: 10.1097/PAS.0b013e31825793ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lidove O., Sedel F., Charlotte F., Froissart R., Vanier M.T. Cirrhosis and liver failure: expanding phenotype of Acid sphingomyelinase-deficient Niemann-Pick disease in adulthood. JIMD Rep. 2015;15:117–121. doi: 10.1007/8904_2014_306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thurberg B.L., Wasserstein M.P., Jones S.A., Schiano T.D., Cox G.F., Puga A.C. Clearance of hepatic sphingomyelin by olipudase Alfa is associated with improvement in lipid profiles in acid sphingomyelinase deficiency. Am J Surg Pathol. 2016;40(9):1232–1242. doi: 10.1097/PAS.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labrune P., Bedossa P., Huguet P., Roset F., Vanier M.T., Odievre M. Fatal liver failure in two children with Niemann-Pick disease type B. J Pediatr Gastroenterol Nutr. 1991;13(1):104–109. doi: 10.1097/00005176-199107000-00020. [DOI] [PubMed] [Google Scholar]
- 70.Chebib N., Thivolet-Bejui F., Cottin V. Interstitial lung disease associated with adult Niemann-Pick disease type B. Respiration. 2017;94(2):237–238. doi: 10.1159/000477465. [DOI] [PubMed] [Google Scholar]
- 71.Freitas H.M.P., Mançano A.D., Rodrigues R.S., et al. Niemann-Pick disease type B: HRCT assessment of pulmonary involvement. J Bras Pneumol. 2017;43(6):451–455. doi: 10.1590/S1806-37562017000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hollak C.E., de Sonnaville E.S., Cassiman D., et al. Acid sphingomyelinase (Asm) deficiency patients in the Netherlands and Belgium: disease spectrum and natural course in attenuated patients. Mol Genet Metab. 2012;107(3):526–533. doi: 10.1016/j.ymgme.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 73.Lidove O., Belmatoug N., Froissart R., et al. [Acid sphingomyelinase deficiency (Niemann-Pick disease type B) in adulthood: a retrospective multicentric study of 28 adult cases] Rev Med Interne. 2017;38(5):291–299. doi: 10.1016/j.revmed.2016.10.387. [DOI] [PubMed] [Google Scholar]
- 74.Capron T., Trigui Y., Gautier C., Puech B., Chanez P., Reynaud-Gaubert M. Respiratory impairment in Niemann-Pick B disease: two case reports and review for the pulmonologist. Respir Med Res. 2019;76:13–18. doi: 10.1016/j.resmer.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Borie R., Crestani B., Guyard A., Lidove O. Interstitial lung disease in lysosomal storage disorders. Eur Respir Rev. 2021;30(160) doi: 10.1183/16000617.0363-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iaselli F., Rea G., Cappabianca S., et al. Adult-onset pulmonary involvement in Niemann-Pick disease type B. Monaldi Arch Chest Dis. 2011;75(4):235–240. doi: 10.4081/monaldi.2011.211. [DOI] [PubMed] [Google Scholar]
- 77.Guillemot N., Troadec C., de Villemeur T.B., Clément A., Fauroux B. Lung disease in Niemann-Pick disease. Pediatr Pulmonol. 2007;42(12):1207–1214. doi: 10.1002/ppul.20725. [DOI] [PubMed] [Google Scholar]
- 78.Thurberg B.L., Diaz G.A., Lachmann R.H., et al. Long-term efficacy of olipudase alfa in adults with acid sphingomyelinase deficiency (ASMD): further clearance of hepatic sphingomyelin is associated with additional improvements in pro- and anti-atherogenic lipid profiles after 42 months of treatment. Mol Genet Metab. 2020;131(1-2):245–252. doi: 10.1016/j.ymgme.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Thurberg B.L., Ganesh J., Giugliani R., et al. Long-term improvements in liver and lipid outcomes in adults and children with acid sphingomyelinase deficiency treated with olipudase alfa enzyme replacement therapy. Hepatology. 2023;78(suppl 1):S195. [Google Scholar]
- 80.Pastores G.M., Weinreb N.J., Aerts H., et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(4):4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Wasserstein M., Bonella F., Giugliani R., et al. Olipudase alfa enzyme replacement therapy reverses interstitial lung disease in adults with acid sphingomyelinase deficiency: long-term pulmonary outcomes of the ASCEND trial. Mol Genet Metab. 2024;141 [Google Scholar]
- 82.Gaudioso Á., Jiang X., Casas J., Schuchman E.H., Ledesma M.D. Sphingomyelin 16 : 0 is a therapeutic target for neuronal death in acid sphingomyelinase deficiency. Cell Death Dis. 2023;14(4):248. doi: 10.1038/s41419-023-05784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents redacted to protect privacy. Details on Sanofi’s data sharing criteria for published and unpublished data and the process for requesting access can be found at: https://www.vivli.org/.