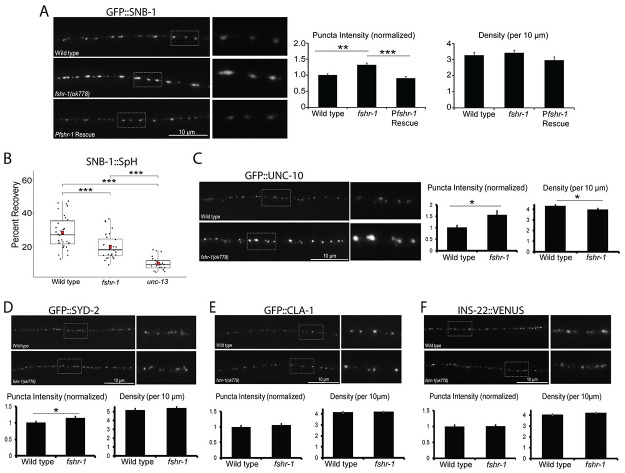
Fig 2. fshr-1 mutants have decreased synaptic vesicle release accompanied by accumulation of some synaptic vesicle and active zone proteins.
(A) Wild type worms, fshr-1(ok778) mutants, and fshr-1 mutants re-expressing fshr-1 genomic DNA under the endogenous fshr-1 promoter (Pfshr-1, agEx43) that also expressed GFP::SNB-1 in cholinergic (ACh) neurons were imaged using a 100x objective. (Left panel) Representative images of the dorsal nerve cords halfway between the vulva and the tail of young adult animals. Boxed areas are shown in higher resolution to the right of the main images. (Right panels) Quantification of puncta (synaptic) intensity and puncta density (per 10 μm) ± s.e.m. Puncta intensity is shown normalized to wild type. For (A), n = 24 animals imaged for wild type, n = 31 for fshr-1, and n = 26 for Pfshr-1 rescue. (B) Percent recovery of SNB-1::Superecliptic pHluorin (SpH) fluorescence at ACh motor neuron presynapses following photobleaching in wild type, fshr-1(ok778), and fusion-defective unc-13(se69) animals. For wild type, n = 30 animals; for fshr-1, n = 28; for unc-13, n = 21. (C-F) Wild type or fshr-1(ok778) mutant animals that also expressed (C) GFP::UNC-10, (D) GFP::SYD-2, (E) GFP::CLA-1, or (F) INS-22::VENUS in ACh neurons were imaged using a 100x objective. (Upper panels) Representative images of the dorsal nerve cords halfway between the vulva and the tail of young adult animals. (Lower panels) Quantification of normalized puncta (synaptic) intensity and puncta density (per 10 μm) ± s.e.m. For (C), n = 25 animals imaged for wild type, n = 24 for fshr-1. For (D), n = 31 for wild type, n = 35 for fshr-1. For (E), n = 30 for wild type, n = 31 for fshr-1. For (F), n = 31 for wild type, n = 35 for fshr-1. One-way ANOVA followed by Tukey’s post hoc tests were used to compare the means of the datasets in A and B; Student’s t tests were used to compare datasets in C-F. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Upper two images and numeric data in (A) originally published in modified format in Hulsey-Vincent et al., 2023a [63].