Abstract
This study aims to investigate the association between the platelet-to-high-density lipoprotein cholesterol ratio (PHR) and diabetes/prediabetes in US adults. Data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES) were analyzed in this study. The PHR was calculated by dividing platelet count by HDL-C concentration. Diabetes and prediabetes were classified according to established clinical criteria. Multivariate logistic regression analyses were employed to estimate odds ratios (ORs) and 95% CIs. We used a two-stage logistic regression model with restricted cubic splines (RCS) to evaluate potential non-linear relationships and to identify inflection points. The discriminative ability of the model was evaluated using receiver operating characteristic (ROC) curves, with the area under the curve (AUC) used to measure model performance. Sensitivity and specificity at the optimal threshold were also reported. Furthermore, subgroup and interaction analyses were conducted to determine variations across different population groups. The study included 20,229 eligible participants, with a mean age of 47.84 years, and 51.80% being female. Among the participants, 14.29% were diagnosed with diabetes, and 44.36% with prediabetes. A positive association was observed between PHR and diabetes/prediabetes. After adjusting for model 3, the OR for the combined outcome of diabetes and prediabetes associated with a per-unit increase in PHR was 1.17 (95% CI: 1.05–1.30). Participants in the highest PHR quartile had an OR of 2.55 (95% CI: 1.52–4.28) compared to those in the lowest quartile. Two-stage regression analysis identified a breakpoint at PHR = 4.55, with a positive association observed when PHR was below this value (OR = 1.33, 95% CI: 1.03–1.70). ROC analysis demonstrated good discriminatory ability of the model, with an AUC of 0.824 (95% CI: 0.803–0.845), sensitivity of 83.2%, and specificity of 66.5%. Stratified analyses revealed significant associations between PHR and diabetes/prediabetes across most demographic groups, with interactions observed for sex, alcohol consumption, and BMI, suggesting these factors may modify the association. This study suggests that an elevated PHR may be associated with a higher likelihood of diabetes and prediabetes. Consequently, PHR could serve as a valuable marker for estimating the association of diabetes and prediabetes development.
Keywords: Diabetes, Prediabetes, Platelet, High-density lipoprotein, PHR, NHANES
Subject terms: Endocrine system and metabolic diseases, Predictive markers
Introduction
Diabetes represents an escalating global health challenge, with its prevalence projected to rise from 9.3% (463 million individuals) in 2019 to 10.9% (700 million) by 20451. Prediabetes, a precursor condition characterized by elevated blood glucose levels, is also exhibiting an upward trend. In 2021, the global prevalence of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) was estimated at 9.1% (464 million) and 5.8% (298 million), respectively. These figures are expected to increase to 10.0% (638 million) for IGT and 6.5% (414 million) for IFG by 20452. This substantial growth in both diabetes and prediabetes cases is anticipated to exacerbate the already significant global health and economic burden. Diabetes is a major contributor to various complications, including cardiovascular disease (CVD), chronic kidney disease (CKD), retinopathy, and neuropathy3–5. In 2019, the global economic burden associated with diabetes was estimated at $1.31 trillion, accounting for over 1.8% of the global GDP6. Consequently, the implementation of targeted strategies to prevent diabetes and prediabetes is crucial for mitigating their prevalence and associated complications.
The platelet-to-high-density lipoprotein cholesterol ratio (PHR) has emerged as a promising indicator, reflecting both platelet activity and HDL-C levels7. Excessive platelet activation is a typical feature of diabetic patients, driven by hyperglycemia, insulin resistance, and chronic inflammation8,9. These factors promote a procoagulant state and impaired fibrinolysis. Activated platelets release various pro-inflammatory and pro-thrombotic mediators, such as thromboxane A2 and platelet factor 4 (PF4), which exacerbate inflammation and further promote insulin resistance, creating a self-perpetuating cycle10–12. HDL-C is renowned for its anti-inflammatory properties and role in lipid metabolism regulation13,14. In diabetic patients, a decrease in HDL-C levels is commonly observed, impairing its ability to clear cholesterol and further exacerbating metabolic imbalances15. This reduction in HDL-C contributes to lipid accumulation, disrupts normal insulin signaling, and worsens insulin resistance16,17. Given the critical roles of both platelet activity and HDL-C in the inflammatory, thrombotic, and lipid dysregulation processes associated with diabetes, PHR emerges as a promising indicator for assessing diabetes development.
PHR was initially introduced by Jialal et al. as an effective biomarker for predicting metabolic syndrome (MetS)7. Subsequent research has also demonstrated that elevated PHR is associated with various metabolic disorders18,19, including hyperuricemia, obesity, and non-alcoholic fatty liver disease (NAFLD). These conditions are characterized by chronic inflammation and dyslipidemia, which are key contributors to diabetes development. These findings underscore the broader role of PHR in metabolic health assessment. However, despite its established relevance in these conditions, the relationship between PHR and diabetes/prediabetes has not been extensively investigated. Therefore, this study aims to address this knowledge gap by utilizing NHANES data to explore the association between PHR and diabetes/prediabetes.
Methods
Study population
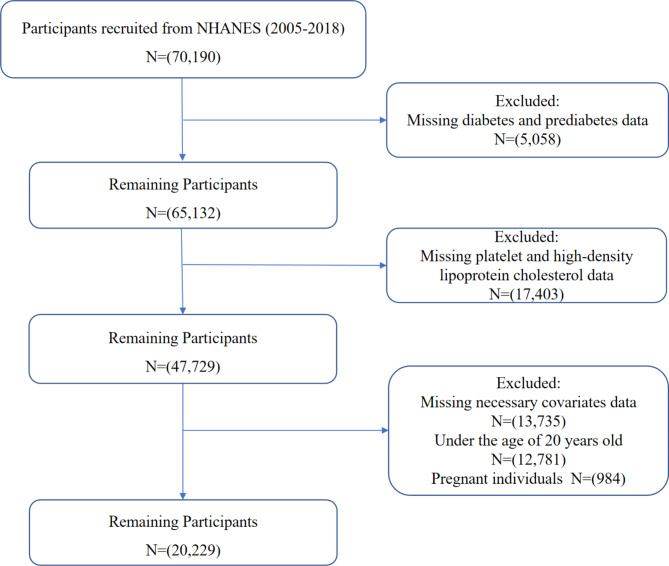
This study utilized data from 2005 to 2018 obtained from the NHANES spanning seven biennial cycles, which conducted a national cross-sectional survey and employed a multistage stratified probability sample. These specific cycles were chosen due to the consistent measurement of the variables required. Among the initially extracted 70,190 participants, exclusions were applied for the following reasons to ensure data accuracy and minimize confounding factors: (1) participants lacking complete data to determine diabetes or prediabetes status (n = 5,058), specifically those missing any diagnostic criteria (fasting blood glucose (FSG), glycosylated hemoglobin (HbA1c), 2-hour oral glucose tolerance test (OGTT), use of diabetes medication or insulin, or self-reported diagnosis); (2) participants missing platelet or HDL-C data (n = 17,403), which are essential for calculating PHR; (3) participants with incomplete covariate information (sex, age, race, poverty income ratio, smoking status, alcohol status, education, and BMI; n = 13,735); (4) individuals under 20 years of age (n = 12,781) and (5) pregnant participants (n = 984). The final sample for analysis included 20,229 individuals. The final analysis included 20,229 individuals, as illustrated in Fig. 1.
Fig. 1.
Flowchart of study participants.
Diagnosis of diabetes and prediabetes
Diabetes was diagnosed in individuals meeting one or more of the following criteria: (1) FSG ≥ 7.0 mmol/L or random blood glucose ≥ 11.1 mmol/L; (2) 2-hour OGTT ≥ 11.1 mmol/L; (3) HbA1c ≥ 6.5%; (4) use of diabetes medication or insulin; or (5) a self-reported diagnosis of diabetes by a healthcare professional.
Prediabetes was diagnosed based on one or more of the following criteria: (1) FSG between 5.6 and 7.0 mmol/L; (2) HbA1c between 5.7 and 6.5%; (3) 2-hour OGTT between 7.8 and 11.1 mmol/L; or (4) a self-reported diagnosis of prediabetes by a healthcare professional.
Calculation of PHR
The PHR was calculated as the ratio of platelet count (1000 cells/µL) to HDL-C (mg/dL)7,18. Blood samples were collected from participants at the Mobile Examination Center by trained personnel for analysis. Platelet counts were obtained using Volume, Conductivity, and Scatter (VCS) technology with Beckman Coulter analyzers. Specifically, the Beckman Coulter MAXM was employed from 2005 to 2012, and the Beckman Coulter DxH 800 was utilized from 2013 to 2018. HDL-C levels were measured using an immunoassay method. The Roche Modular P chemistry analyzer was used from 2005 to 2012, while both the Roche Modular P and Roche Cobas 6000 chemistry analyzers were employed from 2013 to 2018.
Covariates
Demographic data were gathered through questionnaire interviews and included variables such as sex (male, female), age, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, other Hispanic, Non-Hispanic Asian, Other), education (below high school, high school, above high school), family poverty income ratio (PIR, < 1.0, 1.0–3.0, > 3.0), alcohol consumption (no drinks, 1–5 drinks/month, 5–10 drinks/month, > 10 drinks/month), BMI (< 25, 25–29, > 29 kg/m²), and smoking status [never (< 100 cigarettes in lifetime), current (≥ 100 cigarettes and currently smoking), former (≥ 100 cigarettes but not currently smoking)]. Physical activity measured as MET-minutes per week, categorized into three groups (< 700, 700-2,400, > 2,400). Complications included hypertension (based on systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, a prior diagnosis, or a history of anti-hypertensive medication use), CVD (self-reported doctor-diagnosed conditions such as coronary heart disease, heart failure (HF), heart attack, stroke, and angina pectoris), and CKD (self-reported doctor-diagnosed). Laboratory covariates encompassed serum insulin, FSG, HbA1c, total triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), high-sensitivity C-reactive protein (hs-CRP), and creatinine (Cr).
Statistical analysis
All statistical analyses were conducted using R version 4.3.320. In accordance with NHANES recommendations, sampling weights were applied to mitigate the purposeful oversampling of certain demographic categories. These weights were utilized to ensure that the results are representative of the U.S. population, adjusting for unequal probabilities of selection, non-response, and post-stratification. Categorical variables were reported as unweighted counts (percentages), while continuous variables were presented as weighted means (standard errors) or medians (interquartile range). Group differences were assessed using the weighted Student’s t-test, Mann-Whitney U test, and Chi-squared test. A two-sided P-value < 0.05 was considered statistically significant.
Multivariate logistic regression analyses were employed to estimate odds ratios (ORs) and 95% CIs. The logistic regression models were classified into categorical and continuous models. For the categorical model, PHR was divided into quartiles, using the lowest quartile as the reference group. Trend tests (p-trend) were conducted using the median PHR in each quartile. Three models were used to assess the association between PHR and diabetes/prediabetes. Model 1 included only PHR as the independent variable. Model 2 adjusted for demographic and lifestyle factors (sex, age, race, PIR, smoking status, alcohol status, education, BMI, and physical activity) to control for key confounders. Model 3 built upon Model 2 by further adjusting for health-related variables (hypertension, CVD, CKD, TG, LDL-C, hs-CRP, SUA, and Cr), to better account for underlying metabolic and cardiovascular conditions. Subgroup and interaction analyses were conducted to determine variations across different population groups. We used a two-stage logistic regression model with restricted cubic splines (RCS) to assess non-linear associations and to identify an inflection point. To further explore potential non-linear relationships more flexibly, we applied a generalized additive logistic model with a smooth curve. The receiver operating characteristic (ROC) curve was generated, and the area under the curve (AUC) was calculated to assess the discriminative ability of the logistic regression model. Furthermore, sensitivity and specificity were reported at the optimal threshold to further illustrate the model’s predictive capabilities.
Results
Study characteristics
Table 1 presents the weighted study characteristics of participants categorized by PHR quartiles. The study included 20,229 participants from the NHANES 2005–2018 survey, with 9,818 males (48.20%) and 10,481 females (51.80%), and an average age of 47.84 ± 17.07 years. Among them, 3,884 (14.29%) were diagnosed with diabetes, 8,863 (44.36%) with prediabetes, 7,493 (32.85%) with hypertension, and 2,207 (8.88%) with CVD. The majority of participants were Non-Hispanic white (65.18%), 14.75% lived in poverty, and 94.97% had a high school diploma or higher. Additionally, 56.58% had never smoked, and 22.31% had never consumed alcohol. The weighted mean (standard error) BMI was 29.30 ± 6.99 kg/m², platelet count was 238.69 ± 60.08 cells/µL, HDL-C was 1.39 ± 0.43 mmol/L, LDL-C was 2.93 ± 0.92 mmol/L, TG was 1.35 ± 1.07 mmol/L, TC was 4.97 ± 1.07 mmol/L, SUA was 320.42 ± 84.06 mmol/L, and Cr was 77.56 ± 30.68 mmol/L. Significant differences were observed across all PHR quartiles for the other variables, except for the number of CVD cases, which showed no significant difference.
Table 1.
Baseline characteristics of study participants stratified by PHR.
| Characteristic | Overall, N = 20,229 | Q1, N = 5,075 (< 3.51) | Q2, N = 5,085 (3.51–4.56) | Q3, N = 5,067 (4.56–5.87) | Q4, N = 5,072 (> 5.87) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 47.84 ± 17.07 | 52.06 ± 17.61 | 48.32 ± 17.51 | 46.23 ± 16.52 | 44.56 ± 15.56 | < 0.001 |
| Sex, n (%) | < 0.001 | |||||
| Female | 10,481(51.80%) | 2,941 (61.92%) | 2,680 (51.65%) | 2,484 (47.95%) | 2,376 (45.28%) | |
| Male | 9,818 (48.20%) | 2,134 (38.08%) | 2,405 (48.35%) | 2,583 (52.05%) | 2,696 (54.72%) | |
| Race, n (%) | < 0.001 | |||||
| Non-Hispanic White | 7,596 (65.18%) | 2,018 (69.66%) | 1,916 (66.85%) | 1,870 (63.77%) | 1,792 (60.16%) | |
| Non-Hispanic Black | 4,454 (10.82%) | 1,351 (12.32%) | 1,120 (10.57%) | 1,051 (10.50%) | 932 (9.86%) | |
| Mexican American | 2,806 (8.78%) | 469 (5.31%) | 687 (8.45%) | 767 (9.64%) | 883 (11.87%) | |
| Other Hispanic | 2,126 (6.35%) | 411 (4.38%) | 508 (5.63%) | 594 (7.47%) | 613 (8.03%) | |
| Non-Hispanic Asian | 2,583 (5.44%) | 678 (5.60%) | 686 (5.57%) | 609 (5.10%) | 610 (5.48%) | |
| Other Race | 734 (3.43%) | 148 (2.73%) | 168 (2.93%) | 176 (3.52%) | 242 (4.59%) | |
| Alcohol status, n (%) | < 0.001 | |||||
| Non-drinker | 4,005 (22.31%) | 996 (21.32%) | 1,008 (21.93%) | 996 (22.37%) | 1,005 (23.73%) | |
| 1-5drinks/month | 6,985(50.60%) | 1,499(41.96%) | 1,724(49.93%) | 1,820(54.22%) | 1,942(57.07%) | |
| 5-10drinks/month | 1,089 (9.35%) | 298 (9.70%) | 286 (8.98%) | 266 (10.19%) | 239 (8.53%) | |
| > 10 drinks/month | 1,890 (17.74%) | 737 (27.02%) | 522 (19.15%) | 344 (13.22%) | 287 (10.70%) | |
| PIR, n (%) | < 0.001 | |||||
| < 1.0 | 3,994 (14.75%) | 859 (11.59%) | 943 (13.87%) | 1,034 (15.52%) | 1,158 (18.22%) | |
| 1.0–3.0 | 7,751 (36.14%) | 1,864 (32.40%) | 1,899 (34.87%) | 1,958 (37.67%) | 2,030 (39.87%) | |
| > 3.0 | 6,604 (49.11%) | 1,859 (56.02%) | 1,768 (51.26%) | 1,590 (46.81%) | 1,387 (41.92%) | |
| Smoke, n (%) | < 0.001 | |||||
| Current | 3,103 (14.91%) | 654 (11.71%) | 665 (12.70%) | 775 (15.38%) | 1,009 (20.13%) | |
| Former | 4,753 (24.65%) | 1,263 (26.71%) | 1,194 (24.25%) | 1,194 (24.85%) | 1,102 (22.68%) | |
| Never | 11,633 (56.58%) | 2,967 (58.15%) | 3,031 (59.24%) | 2,888 (55.51%) | 2,747 (53.24%) | |
| NA | 810 (3.86%) | 191 (3.43%) | 195 (3.82%) | 210 (4.26%) | 214 (3.96%) | |
| Education, n (%) | < 0.001 | |||||
| Below high school | 1,914 (4.98%) | 423 (4.07%) | 457 (4.71%) | 518 (5.39%) | 516 (5.78%) | |
| High school | 7,046 (31.72%) | 1,610 (27.10%) | 1,736 (30.42%) | 1,777 (33.11%) | 1,923 (36.55%) | |
| Above high school | 11,319 (63.25%) | 3,036 (68.78%) | 2,887 (64.80%) | 2,767 (61.45%) | 2,629 (57.65%) | |
| NA | 20(< 0.1%) | 6(< 0.1%) | 5(< 0.1%) | 5(< 0.1%) | 4(< 0.1%) | |
| Physical activity (MET-mins/week), n (%) | < 0.001 | |||||
| < 700 | 3,430(16.96%) | 654 (12.89%) | 723 (14.23%) | 760 (15.00%) | 1,293 (25.49%) | |
| 700 − 2,400 | 6,128(30.29%) | 1,410 (27.78%) | 1,435 (28.22%) | 1,569 (30.97%) | 1,709 (33.69%) | |
| > 2,400 | 7,843 (38.77%) | 2,180 (42.96%) | 2,046 (40.24%) | 1,934 (38.17%) | 1683 (33.18%) | |
| NA | 2,828(13.98%) | 831 (16.37%) | 881 (17.31%) | 804 (15.86%) | 387 (7.64%) | |
| CVD, n (%) | 2,207 (8.88%) | 615 (9.56%) | 558 (8.76%) | 504 (8.18%) | 530 (9.04%) | 0.3 |
| Hypertension, n (%) | 7,493 (32.85%) | 1,835 (30.64%) | 1,841 (31.54%) | 1,827 (33.04%) | 1,990 (36.35%) | < 0.001 |
| Diabetes, n (%) | 3,884 (14.29%) | 747 (9.829%) | 910 (13.12%) | 1,020 (14.69%) | 1,207 (19.82%) | < 0.001 |
| Prediabetes, n (%) | 8,863 (44.36%) | 2,090 (39.01%) | 2,186 (42.54%) | 2,236 (45.36%) | 2,351 (51.36%) | < 0.001 |
| CKD, n (%) | 576 (3.03%) | 147 (3.55%) | 148 (3.13%) | 134 (2.59%) | 147 (2.83%) | < 0.001 |
| BMI, kg/m2 | 29.30 ± 6.99 | 26.44 ± 5.78 | 28.37 ± 6.35 | 30.26 ± 6.97 | 32.31 ± 7.39 | < 0.001 |
| Platelet, 1000 cells/µL | 238.69 ± 60.08 | 194.79 ± 43.31 | 223.65 ± 41.49 | 248.07 ± 45.45 | 291.15 ± 62.60 | < 0.001 |
| HDL-C, mmol/L | 1.39 ± 0.43 | 1.84 ± 0.45 | 1.44 ± 0.27 | 1.24 ± 0.23 | 1.03 ± 0.21 | < 0.001 |
| LDL-C, mmol/L | 2.93 ± 0.92 | 2.80 ± 0.88 | 2.94 ± 0.91 | 3.02 ± 0.92 | 2.97 ± 0.94 | < 0.001 |
| TG, mmol/L | 1.35 ± 1.07 | 0.93 ± 0.51 | 1.22 ± 0.79 | 1.48 ± 0.97 | 1.91 ± 1.60 | < 0.001 |
| TC, mmol/L | 4.97 ± 1.07 | 5.09 ± 1.03 | 4.93 ± 1.03 | 4.96 ± 1.07 | 4.91 ± 1.14 | < 0.001 |
| SUA, mmol/L | 320.42 ± 84.06 | 302.15 ± 79.54 | 312.93 ± 80.56 | 328.06 ± 84.99 | 339.56 ± 86.32 | < 0.001 |
| Sr, umol/L | 77.56 ± 30.68 | 77.30 ± 36.54 | 77.46 ± 30.05 | 77.79 ± 23.99 | 77.70 ± 30.76 | < 0.001 |
| hs-CRP, mg/L | 3.89 ± 7.24 | 2.50 ± 4.80 | 3.15 ± 6.03 | 3.95 ± 5.90 | 6.05 ± 10.50 | < 0.001 |
Normally distributed continuous variables are described as means ± SEs, and continuous variables without a normal distribution are described as medians (interquartile ranges). Categorical variables are presented as numbers (percentages). All estimatess accounted for complex survey designs.
Association between PHR and the diabetes/prediabetes
A significant correlation was observed between higher PHR quartiles and increased odds of diabetes and prediabetes compared to the lowest quartile across all three models (Table 2). After adjusting for all covariates, the Adjusted OR (95% CI) was 1.89 (1.24–2.87, P < 0.05) for Q2, 2.18 (1.30–3.64, P < 0.05) for Q3, and 2.55 (1.52–4.28, P < 0.05) for Q4. When PHR was analyzed as a continuous variable, positive associations were also observed, with Adjusted ORs (95% CI) of 1.13 (1.11–1.16, P < 0.05) in Model 1, 1.13 (1.08–1.16, P < 0.05) in Model 2, and 1.17 (1.05–1.30, P < 0.05) in Model 3. Separate analyses for diabetes and prediabetes further confirmed the positive association with PHR. For diabetes, the Adjusted OR (95% CI) for PHR as a continuous variable was 1.13 (1.09–1.17, P < 0.05), while participants in the highest PHR quartile had an Adjusted OR (95% CI) of 2.08 (1.66–2.61, P < 0.05) compared to the lowest quartile. Similarly, for prediabetes, the Adjusted OR (95% CI) for PHR as a continuous variable was 1.18 (1.06–1.32, P < 0.05), and the highest quartile exhibited an Adjusted OR (95% CI) of 2.68 (1.61–4.45, P < 0.05) relative to the lowest quartile.
Table 2.
Adjusted OR (95% CI) for the associations between phr and diabetes/prediabetes.
| PHR | Range | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Diabetes | ||||
| Continuous | 1.15 (1.12, 1.18) | 1.14 (1.10, 1.18) | 1.13 (1.09, 1.17) | |
| Categories | ||||
| Q1 | < 3.51 | 1 | 1 | 1 |
| Q2 | 3.51–4.56 | 1.39 (1.19, 1.61) | 1.35 (1.09, 1.68) | 1.32 (1.06, 1.64) |
| Q3 | 4.56–5.87 | 1.58 (1.36, 1.83) | 1.46 (1.18, 1.82) | 1.42 (1.14, 1.77) |
| Q4 | > 5.87 | 2.27 (1.96, 2.62) | 2.18 (1.74, 2.73) | 2.08 (1.66, 2.61) |
| p-trend | < 0.01 | < 0.01 | < 0.01 | |
| Prediabetes | ||||
| Continuous | 1.09 (1.07, 1.12) | 1.11 (1.08, 1.15) | 1.18 (1.06, 1.32) | |
| Categories | ||||
| Q1 | < 3.51 | 1 | 1 | 1 |
| Q2 | 3.51–4.56 | 1.16 (1.03, 1.30) | 1.29 (1.10, 1.51) | 2.09 (1.38, 3.17) |
| Q3 | 4.56–5.87 | 1.30 (1.16, 1.46) | 1.43 (1.21, 1.69) | 2.38 (1.43, 3.98) |
| Q4 | > 5.87 | 1.65 (1.47, 1.85) | 1.79 (1.50, 2.12) | 2.68 (1.61, 4.45) |
| p-trend | < 0.01 | < 0.01 | < 0.01 | |
| Diabetes and Prediabetes | ||||
| Continuous | 1.13 (1.11, 1.16) | 1.13 (1.08, 1.16) | 1.17 (1.05, 1.30) | |
| Categories | ||||
| Q1 | < 3.51 | 1 | 1 | 1 |
| Q2 | 3.51–4.56 | 1.21 (1.08, 1.35) | 1.26 (1.09, 1.47) | 1.89 (1.24, 2.87) |
| Q3 | 4.56–5.87 | 1.39 (1.25, 1.56) | 1.44 (1.20, 1.68) | 2.18 (1.30, 3.64) |
| Q4 | > 5.87 | 1.93 (1.73, 2.16) | 1.88 (1.60, 2.21) | 2.55 (1.52, 4.28) |
| p-trend | < 0.01 | < 0.01 | < 0.01 | |
The PHR was categorized into four quartiles and tests for trend (p–trend) based on variable containing the median value for each quartiles. PHR also was utilized as continuous variables and p-value was used to test significance.
Model 1 included only PHR as the independent variable. Model 2 included adjustments for sex, race, age, poverty status, smoking status, alcohol status, education, BMI, and physical activity. Model 3 built on Model 2 by further adjusting for hypertension, CVD, CKD, TG, LDL-C, hs-CRP, SUA and Cr.
Figure 2 illustrates the nonlinear relationship between PHR and diabetes/prediabetes after full adjustments, modeled using a smoothed curve from a generalized additive logistic model (P for nonlinearity < 0.01). A two-stage logistic regression analysis identified an inflection point at 4.55 (Table 3). The results revealed that for PHR < 4.55, the Adjusted OR was 1.33 (95% CI: 1.03–1.70, P < 0.05), while for PHR ≥ 4.55, the Adjusted OR was 1.06 (95% CI: 0.93–1.25, P > 0.05). These findings suggest that below the inflection point, a lower PHR is associated with decreased odds of diabetes/prediabetes. In the overall study population, 55.03% of participants had a PHR below 4.55. This relatively even distribution around the inflection point underscores the significance of 4.55 as a critical threshold. Subgroup analysis revealed that 50.17% of males and 58.49% of females had a PHR < 4.55, indicating a higher proportion of females with PHR below 4.55 compared to males.
Fig. 2.
RCS analysis of PHR with diabetes and prediabetes based on model 3 with comprehensive adjustments.
Table 3.
Threshold analysis of the effect of PHR on diabetes and prediabetes using two-stage logistic regression models.
| Diabetes and prediabetes | Adjusted OR (95% CI) | P–value |
|---|---|---|
| Fitting by binary logistic regression model | 1.17 (1.05, 1.30) | 0.04 |
| Fitting by the two–stage logistic regression model | ||
| Inflection point | 4.55 | |
| PHR < 4.55 | 1.33 (1.03, 1.70) | 0.02 |
| PHR ≥ 4.55 | 1.06 (0.93, 1.25) | 0.13 |
| Log–likelihood ratio | < 0.001 | |
Analyses were based on Model 3 with comprehensive adjustments, including adjustments for sex, race, age, poverty status, smoking status, alcohol status, education, BMI, physical activity, hypertension, CVD, CKD, TG, LDL-C, hs-CRP, SUA, and Cr.
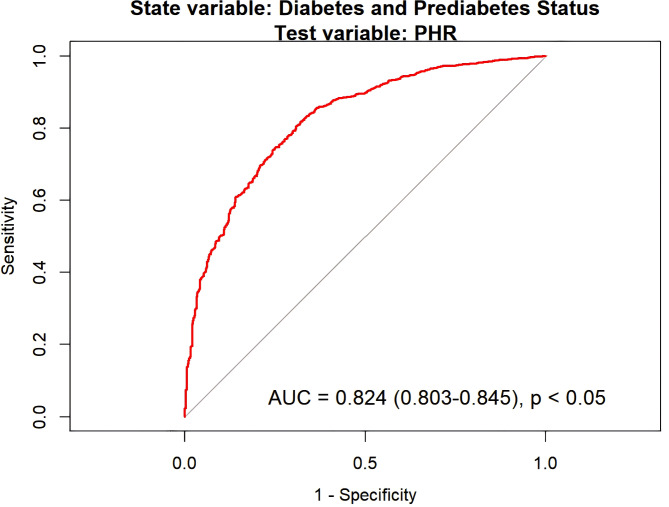
ROC analysis (Fig. 3) demonstrated that PHR possesses good discriminatory ability in predicting diabetes and prediabetes, with an AUC of 0.824 (95% CI: 0.803–0.845, P < 0.05) in the fully adjusted Model 3. The optimal threshold was determined to be 0.6551 by maximizing the Youden index. At this threshold, the model achieved a sensitivity of 83.2% and a specificity of 66.5%. These results indicate that PHR provides high accuracy at this threshold.
Fig. 3.
ROC curve for PHR in predicting diabetes and prediabetes based on model 3 with comprehensive adjustments.
Stratified assessment
Stratified analyses were conducted to evaluate the influence of confounding factors and specific population subgroups on the results. As presented in Table 4, significant associations between PHR and diabetes/prediabetes were observed across the majority of demographic groups. However, Mexican Americans and Other Hispanics demonstrated no significant association (P > 0.05). These findings suggest that the majority of the examined subgroups may exhibit higher odds of diabetes/prediabetes. (P < 0.05).
Table 4.
Subgroup analyses of the association between PHR and diabetes/prediabetes.
| PHR | Continuous | Q1 | Q2 | Q3 | Q4 | P for trend | P for interaction |
|---|---|---|---|---|---|---|---|
| Age | 0.96 | ||||||
| < 60 | 1.10(1.03,1.16) | 1 | 1.18(0.91,1.54) | 1.12(0.83,1.51) | 1.95(1.38,2.75) | < 0.001 | |
| ≥ 60 | 1.10(1.06,1.13) | 1 | 1.19(0.98,1.44) | 1.35(1.12,1.65) | 1.57(1.30,1.90) | < 0.001 | |
| Sex | < 0.01 | ||||||
| Male | 1.07(1.03,1.12) | 1 | 1.02(0.86,1.21) | 1.22(1.04,1.45) | 1.46(1.24,1.73) | < 0.001 | |
| Female | 1.20(1.16,1.25) | 1 | 1.32(1.06,1.64) | 1.60(1.28,2.04) | 2.34(1.85,2.97) | < 0.001 | |
| Race | 0.37 | ||||||
| Non-Hispanic White | 1.13(1.08,1.17) | 1 | 1.32(1.07,1.63) | 1.45(1.17,1.83) | 1.99(1.57,2.49) | < 0.001 | |
| Non-Hispanic Black | 1.13(1.06,1.19) | 1 | 1.02(0.79,1.32) | 1.42(1.08,1.84) | 1.93(1.44,2.56) | < 0.001 | |
| Mexican American | 1.14(1.05,1.22) | 1 | 0.67(0.45,1.01) | 0.95(0.64,1.42) | 1.34(0.90,1.98) | < 0.001 | |
| Other Hispanic | 1.13(1.04,1.21) | 1 | 1.00(0.65,1.56) | 1.20(0.76,1.88) | 1.50(0.99,2.33) | < 0.001 | |
| Non-Hispanic Asian | 1.14(1.06,1.22) | 1 | 1.85(1.28,2.67) | 1.32(0.89,1.96) | 1.75(1.16,2.65) | < 0.001 | |
| Education | 0.16 | ||||||
| Below high school | 1.15(1.03,1.27) | 1 | 1.03(0.60,1.77) | 1.04(0.61,1.78) | 1.99(1.16,3.37) | < 0.001 | |
| High school | 1.14(1.09,1.20) | 1 | 1.22(0.92,1.61) | 1.43(1.08,1.89) | 2.00(1.50,2.63) | < 0.001 | |
| Above high school | 1.12(1.08,1.16) | 1 | 1.29(1.05,1.58) | 1.44(1.17,1.78) | 1.80(1.45,2.23) | < 0.001 | |
| Smoke | 0.06 | ||||||
| Current | 1.17(1.09,1.25) | 1 | 1.90(1.24,2.90) | 1.96(1.26,3.04) | 2.71(1.77,4.13) | < 0.001 | |
| Former | 1.12(1.03,1.20) | 1 | 1.07(0.79,1.47) | 1.60(1.15,2.24) | 2.08(1.45,2.98) | < 0.001 | |
| Never | 1.12(1.07,1.16) | 1 | 1.15(0.94,1.41) | 1.15(0.92,1.42) | 1.74(1.24,2.01) | < 0.001 | |
| Alcohol status, n (%) | 0.02 | ||||||
| Non-drinker | 1.11(1.05,1.17) | 1 | 1.28(0.94,1.75) | 1.42(1.05,1.96) | 1.62(1.19,2.20) | < 0.001 | |
| 1-5drinks/month | 1.12(1.07,1.17) | 1 | 1.03(0.82,1.30) | 1.16(0.91,1.47) | 1.70(1.34,2.17) | < 0.001 | |
| 5-10drinks/month | 1.17(1.06,1.32) | 1 | 1.44(0.84,2.48) | 2.40(1.37,4.32) | 2.51(1.39,4.53) | < 0.001 | |
| > 10 drinks/month | 1.18(1.07,1.35) | 1 | 1.73(1.21,2.46) | 1.65(1.11,2.45) | 2.30(1.48,3.95) | < 0.001 | |
| PIR, n (%) | 0.30 | ||||||
| < 1.0 | 1.15(1.09,1.21) | 1 | 1.46(1.05,2.03) | 1.47(1.07,2.02) | 2.08(1.49,2.88) | < 0.001 | |
| 1.0–3.0 | 1.13(1.08,1.18) | 1 | 1.20(0.93,1.54) | 1.47(1.15,1.91) | 1.87(1.45,2.42) | < 0.001 | |
| > 3.0 | 1.14(1.09,1.19) | 1 | 1.26(0.99,1.59) | 1.36(1.06,1.77) | 1.82(1.40,2.38) | < 0.001 | |
| Physical activity, n (%) | 0.25 | ||||||
| < 700 | 1.14(1.08,1.21) | 1 | 1.26(1.15,2.00) | 1.31(1.17,2.02) | 1.89(1.34,2.17) | < 0.001 | |
| 700 − 2,400 | 1.16(1.10,1.20) | 1 | 1.39(1.23,2.11) | 1.44(1.35,2.19) | 2.23(1.48,2.72) | < 0.001 | |
| > 2,400 | 1.15(1.08,1.19) | 1 | 1.29(1.18,1.79) | 1.46(1.16,2.07) | 2.18(1.40,2.48) | < 0.001 | |
| BMI | 0.02 | ||||||
| < 25 | 1.06(1.04,1.14) | 1 | 1.36(1.05,1.77) | 1.64(1.21,2.23) | 2.13(1.53,2.98) | < 0.001 | |
| ≥ 25 | 1.13(1.05,1.18) | 1 | 1.29(1.06,1.56) | 1.53(1.26,1.85) | 2.20(1.81,2.66) | < 0.001 | |
| Hypertension, n (%) | 0.90 | ||||||
| 1 | 1.13(1.07,1.18) | 1 | 1.55(1.19,2.03) | 1.55(1.18,2.04) | 2.00(1.52,2.66) | < 0.001 | |
| 0 | 1.13(1.08,1.18) | 1 | 1.14(0.94,1.39) | 1.37(1.12,1.67) | 1.85(1.46,2.23) | < 0.001 | |
| CVD, n (%) | 0.30 | ||||||
| 1 | 1.10(1.01,1.18) | 1 | 2.12(1.27,3.53) | 1.18(0.73,1.94) | 1.85(1.05,3.26) | < 0.001 | |
| 0 | 1.13(1.09,1.17) | 1 | 1.22(1.03,1.44) | 1.44(1.21,1.71) | 1.86(1.56,2.22) | < 0.001 | |
Analyses were based on Model 3 with comprehensive adjustments, including adjustments for sex, race, age, poverty status, smoking status, alcohol status, education, BMI, physical activity, hypertension, CVD, CKD, TG, LDL-C, hs-CRP, SUA, and Cr.
The impact of various patient characteristics on the observed associations was also investigated. These characteristics included age, sex, race, BMI, education level, smoking status, alcohol consumption, PIR, physical activity, hypertension, and CVD. Significant interactions were identified with sex, alcohol consumption, and BMI (P for interaction < 0.05). These results indicate that these specific factors may play a moderating role in the association between PHR and diabetes/prediabetes.
Discussion
This study unveils a robust positive association between PHR and diabetes/prediabetes. The association remains statistically significant even after accounting for a wide array of potential confounding factors. Following comprehensive adjustment, the OR for diabetes and prediabetes per-unit increase in PHR was determined to be 1.17 (95% CI: 1.05–1.30, P < 0.05). Notably, participants in the highest PHR quartile exhibited an OR of 2.55 (95% CI: 1.52–4.48, P < 0.05) when compared to those in the lowest quartile. The consistency of this positive correlation across various demographic groups was further substantiated through subgroup analyses and interaction tests. Additionally, RCS analysis revealed a non-linear relationship between PHR and diabetes/prediabetes. A two-stage regression analysis revealed an inflection point at PHR = 4.55, with a stronger positive association with diabetes and prediabetes observed when PHR is below this threshold. The discriminatory ability of PHR was evaluated using ROC analysis. The AUC for PHR was calculated to be 0.824, demonstrating good discriminatory power. At the optimal threshold, the sensitivity and specificity were determined to be 83.2% and 66.5%, respectively. These findings provide strong support for the potential utility of PHR as a clinical screening tool for diabetes and prediabetes.
The subgroup analysis revealed significant interactions between sex, drinking status, and BMI in the relationship between PHR and diabetes/prediabetes. Firstly, a stronger positive correlation was observed in males compared to females, potentially attributable to higher levels of inflammation and platelet activation associated with metabolic disorders in men21. Conversely, the generally higher HDL-C levels in women may exert a more pronounced protective effect against diabetes, thereby attenuating the impact of PHR22. Secondly, the positive correlation was found to be more robust among heavy drinkers, which may be attributed to the complex effects of alcohol on lipid metabolism and inflammation. Previous studies have suggested that moderate alcohol consumption may reduce diabetes risk by increasing HDL-C levels and reducing inflammation. However, excessive alcohol intake can exacerbate metabolic disorders, thereby enhancing the predictive power of PHR23,24. Lastly, the positive correlation was more pronounced in individuals with high BMI. Obesity is frequently accompanied by chronic inflammation, insulin resistance, and lipid abnormalities25, all of which amplify the influence of PHR on diabetes and prediabetes.
In recent years, PHR has garnered attention as a novel biomarker in metabolic disease research7. Study has demonstrated that PHR is significantly elevated in patients with MetS and increases with MetS severity7. Additionally, a positive correlation between PHR and the risk of hyperuricemia has been reported18. MetS is characterized by a constellation of metabolic disorders centered on insulin resistance, which are closely associated with diabetes development26,27. Furthermore, hyperuricemia, beyond being a component of MetS, is an independent risk factor for insulin resistance and diabetes progression28–30. Large-scale prospective cohort studies have confirmed that MetS and hyperuricemia significantly increase the risk of developing diabetes31,32. Consequently, it is logical to postulate that PHR is closely related to diabetes or prediabetes. Moreover, recent investigations have expanded the clinical applications of PHR by exploring its association with other conditions such as NAFLD19, kidney stones33, depression34, and HF35. For instance, research has demonstrated that elevated PHR is associated with an increased risk of kidney stones, highlighting the role of platelet activity and inflammation in renal pathologies. Another study has identified a correlation between PHR and HF, with the underlying mechanism possibly related to systemic inflammation and vascular dysfunction. These findings suggest that PHR is not only relevant to metabolic syndrome and diabetes but may also serve as a marker of systemic inflammation and metabolic dysregulation in a broader range of conditions. The present study addresses a gap in the field by specifically demonstrating the association of PHR with diabetes and prediabetes, while also exploring the underlying mechanisms of this association.
The association between PHR and diabetes is mediated by complex pathophysiological mechanisms that remain incompletely elucidated. This paper explores these connections by focusing on three key factors: chronic inflammation, oxidative stress, and lipid metabolism disorders.
Inflammatory responses play a crucial role in the pathogenesis of diabetes, with platelets serving as key mediators in this process36,37. Activated platelets not only participate in coagulation but also release various pro-inflammatory mediators, such as PF4, transforming growth factor-β (TGF-β), and platelet-activating factor (PAF), which trigger and sustain chronic low-grade inflammation38–40. These pro-inflammatory mediators interact with immune cells, such as monocytes and macrophages, activating them and promoting further cytokine release, thus creating an inflammatory cascade41. Studies have demonstrated that a chronic inflammatory state directly impairs insulin signaling pathways. This impairment leads to increased insulin resistance, which is a fundamental pathological characteristic of diabetes36,40. Additionally, the interaction between platelets and leukocytes results in the formation of platelet-leukocyte aggregates, which further amplify the pro-inflammatory activity of leukocytes. These aggregates play a significant role in the inflammatory response that occurs within atherosclerotic plaques, directly contributing to the exacerbation of insulin resistance and the impairment of blood glucose regulation42. HDL-C is recognized for its anti-inflammatory properties, capable of reducing inflammatory responses through various mechanisms. These mechanisms include promoting reverse cholesterol transport, inhibiting the oxidation of LDL-C, and clearing circulating pro-inflammatory factors43,44. An elevated PHR may indicate an increase in the pro-inflammatory effects of platelets and a reduction in the anti-inflammatory effects of HDL-C. This imbalance exacerbates chronic inflammation, potentially leading to increased insulin resistance and the development of diabetes.
Pancreatic β cells are particularly susceptible to damage by reactive oxygen species (ROS) due to their relatively weak antioxidant capacity. Research has shown that excessive platelet activation increases oxidative stress by producing ROS, which not only damages vascular endothelial cells but also pancreatic β cells, thereby compromising their ability to secrete insulin45–47. Platelets in diabetic patients are often in a highly activated state, which is closely linked to heightened oxidative stress48. HDL-C possesses antioxidant properties and can protect pancreatic β-cells by scavenging excess ROS49. Therefore, the imbalance between oxidative stress and the antioxidant system could be one of the key mechanisms underlying the association between PHR and diabetes.
Lipid metabolism disorders constitute a crucial pathological basis for the development of diabetes, with HDL-C playing a critical role in this process. Firstly, HDL-C facilitates reverse cholesterol transport, aiding in the clearance of excess cholesterol from peripheral tissues, thereby reducing the risk of atherosclerosis50. In diabetic patients, HDL-C levels are often significantly reduced, which directly impairs its protective role in maintaining lipid metabolism balance51,52. The reduction in HDL-C levels is not only associated with the formation of atherosclerotic plaques but also disrupts insulin signaling pathways, thereby exacerbating insulin resistance53,54. Secondly, low HDL-C levels are usually accompanied by elevated TG and LDL-C, resulting in a more pronounced state of dyslipidemia, which further exacerbates insulin resistance. Several studies53,55 have demonstrated that this combination disrupts normal lipid homeostasis, leading to increased fat deposition in peripheral tissues and weakening insulin’s ability to facilitate glucose uptake. Consequently, the worsening lipid profile accelerates the progression of metabolic dysfunction, contributing to the development of diabetes.
The present study exhibits several major strengths. Firstly, it is the first study to utilize a nationally representative sample to examine the associations of the PHR with diabetes and prediabetes. Secondly, a wide array of potential confounding variables was accounted for in the analysis. Thirdly, the accuracy and reliability of the data were bolstered by employing trained staff who adhered to standardized protocols for collecting key information and conducting participant interviews.
However, this study also has some limitations that should be acknowledged. Firstly, as a cross-sectional study, causal relationships cannot be established. Additionally, the study’s capacity to explore and test etiological hypotheses is limited, and its findings may not be fully generalizable. Therefore, further prospective longitudinal studies are needed to confirm these findings. Secondly, potential confounding from unknown or unmeasurable factors cannot be entirely excluded. Thirdly, due to the presence of randomly missing data and the large sample size, multiple imputation methods were not used to address the missing data, which may impact the precision of the results.
Conclusions
This study suggests that an elevated PHR may be associated with a higher likelihood of diabetes and prediabetes. Consequently, PHR could serve as a valuable marker for estimating the association of diabetes and prediabetes development.
Acknowledgements
We would like to appreciate the support by participants involved in the NHANES study.
Author contributions
Jianpeng Du and Dazhuo Shi: conceptualization and supervision. Pengfei Chen and Meilin Zhu: data curation and writing the original draft. Ming Guo and Zhuhong Chen: editing and final revisions. All authors contributed to and approved the submitted version of the article.
Funding
This work was supported by the Project of Hospital capability enhancement project of Xiyuan Hospital, CACMS (NO. XYZX0204-02, NO. XYZX0201-03); the Project of Scientific and technological innovation project of China Academy of Chinese Medical Sciences (NO. CI2021A01618).
Data availability
The original contributions presented in this study are included in the article. For further inquiries, please contact the corresponding author, Jianpeng Du, at 13811518062@163.com.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the NHANES Institutional Review Board, and was performed in accordance with the Declaration of Helsinki, with all NHANES participants providing signed informed consent. Details are available at https://www.cdc.gov/nchs/nhanes/irba98.htm.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengfei Chen, Meilin Zhu and Ming Guo contributed equally to this work.
Contributor Information
Zhuhong Chen, Email: 13264214919@163.com.
Jianpeng Du, Email: 13811518062@163.com.
References
- 1.Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9 edition. Diabetes Res. Clin. Pract.157, 107843 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sánchez, E. et al. Characteristics of atheromatosis in the prediabetes stage: A cross-sectional investigation of the ILERVAS project. Cardiovasc. Diabetol.18(1), 154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin, X. et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep.10(1), 14790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2021 Fertility and Forecasting Collaborators. Global fertility in 204 countries and territories, 1950–2021, with forecasts to 2100: A comprehensive demographic analysis for the global burden of Disease Study 2021. Lancet403(10440), 2057–2099 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bragg, F. et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA317(3), 280–289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommer, C. et al. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol.5(6), 423–430 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Jialal, I., Jialal, G. & Adams-Huet, B. The platelet to high density lipoprotein -cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes Metab. Res. Rev.37(6), e3403 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Davì, G. et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation99(2), 224–229 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Al-Sofiani, M. E. et al. Diabetes and platelet response to low-dose aspirin. J. Clin. Endocrinol. Metab.103(12), 4599–4608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin, G. et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci. Rep.6, 36222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajos, G. et al. Polyhedrocytes in blood clots of type 2 diabetic patients with high cardiovascular risk: Association with glycemia, oxidative stress and platelet activation. Cardiovasc. Diabetol.17(1), 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, A. M. et al. Salvianolic acid a inhibits platelet activation and aggregation in patients with type 2 diabetes mellitus. BMC Cardiovasc. Disord.20(1), 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan, L. W. et al. High levels of LDL-C combined with low levels of HDL-C further increase platelet activation in hypercholesterolemic patients. Braz J. Med. Biol. Res.48(2), 167–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flierl, U. & Schäfer, A. Fractalkine–a local inflammatory marker aggravating platelet activation at the vulnerable plaque. Thromb. Haemost.108(3), 457–463 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Zheng, Y. Y. et al. Low HDL cholesterol is associated with reduced bleeding risk in patients who underwent PCI: Findings from the PRACTICE study. Thromb. Haemost (2023). [DOI] [PubMed]
- 16.Gao, Y. et al. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: Evidence from a large national cohort study. Lipids Health Dis.23(1), 66 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohrabi, Y., Schwarz, D. & Reinecke, H. LDL-C augments whereas HDL-C prevents inflammatory innate immune memory. Trends Mol. Med.28(1), 1–4 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Yan, L. et al. Association of platelet to high-density lipoprotein cholesterol ratio with hyperuricemia. Sci. Rep.14(1), 15641 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu, C. F. et al. Association between the platelet/high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: Results from NHANES 2017–2020. Lipids Health Dis.22(1), 130 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024). https://www.R-project.org/
- 21.Hadley, J. B. et al. Hormones, age, and sex affect platelet responsiveness in vitro. Transfusion62(9), 1882–1893 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holven, K. B. & van Roeters, J. Sex differences in lipids: A life course approach. Atherosclerosis384, 117270 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Geng, T. et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PLoS Med.20(1), e1004135 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han, T. et al. Eighteen-year alcohol consumption trajectories and their association with risk of type 2 diabetes and its related factors: The China Health and Nutrition Survey. Diabetologia62(6), 970–980 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Piché, M. E., Tchernof, A. & Després, J. P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res.126(11), 1477–1500 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Hudish, L. I., Reusch, J. E. & Sussel, L. β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Invest.129(10), 4001–4008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaisetthawatkul, P. et al. Prediabetes, diabetes, metabolic syndrome, and small fiber neuropathy. Muscle Nerve. 61(4), 475–479 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Jiang, J. et al. Prevalence of diabetes in patients with hyperuricemia and gout: A systematic review and meta-analysis. Curr. Diab Rep.23(6), 103–117 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Mortada, I. Hyperuricemia, Type 2 diabetes Mellitus, and hypertension: An emerging association. Curr. Hypertens. Rep.19(9), 69 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Vareldzis, R., Perez, A. & Reisin, E. Hyperuricemia: An intriguing connection to metabolic syndrome, diabetes, kidney disease, and hypertension. Curr. Hypertens. Rep.26(6), 237–245 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Woyesa, S. B., Hirigo, A. T. & Wube, T. B. Hyperuricemia and metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC Endocr. Disord.17(1), 76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstock, R. S. et al. Metabolic syndrome is common and persistent in youth-onset type 2 diabetes: Results from the TODAY clinical trial. Obes. (Silver Spring). 23(7), 1357–1361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni, J. et al. Associations between the platelet/high-density lipoprotein cholesterol ratio and likelihood of nephrolithiasis: A cross-sectional analysis in United States adults. Front. Endocrinol. (Lausanne)15, 1289553 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni, J. et al. Examining the cross-sectional relationship of platelet/high-density lipoprotein cholesterol ratio with depressive symptoms in adults in the United States. BMC Psychiatry. 24(1), 427 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, B. et al. The potential of platelet to high-density lipoprotein cholesterol ratio (PHR) as a novel biomarker for heart failure. Sci. Rep.14(1), 23283 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esser, N. et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract.105(2), 141–150 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Tong, H. V. et al. Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus. J. Diabetes Investig. 8(3), 295–305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig, N., Hilger, A., Zarbock, A. & Rossaint, J. Platelets at the crossroads of pro-inflammatory and resolution pathways during inflammation. Cells11(12), 1957 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrell, C. N. et al. Emerging roles for platelets as immune and inflammatory cells. Blood123(18), 2759–2767 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunjungputri, R. N. et al. Differential effects of platelets and platelet inhibition by ticagrelor on TLR2- and TLR4-mediated inflammatory responses. Thromb. Haemost. 113(5), 1035–1045 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Gruden, G. et al. Plasma beta-thromboglobulin and platelet factor 4 are not increased in insulin-dependent diabetic patients with microalbuminuria. Acta Diabetol.31(3), 130–132 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Hafner, C. et al. Brief high oxygen concentration induces oxidative stress in leukocytes and platelets: A randomized cross-over pilot study in healthy male volunteers. Shock56(3), 384–395 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Zhang, J. et al. The mediation effect of HDL-C: Non-HDL-C on the association between inflammatory score and recurrent coronary events. Heliyon10(1), e23731 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolahi Ahari, R. et al. Association of three novel inflammatory markers: Lymphocyte to HDL-C ratio, high-sensitivity C-reactive protein to HDL-C ratio and high-sensitivity C-Reactive protein to lymphocyte ratio with metabolic syndrome. Endocrinol. Diabetes Metab.7(3), e00479 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, S. et al. Matrine impairs platelet function and thrombosis and inhibits ROS production. Front. Pharmacol.12, 717725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han, M. et al. Luteolin protects pancreatic β cells against apoptosis through regulation of autophagy and ROS clearance. Pharmaceuticals (Basel)16(7), 975 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang, Q. et al. Selenium Nanodots (SENDs) as antioxidants and antioxidant-prodrugs to rescue islet β cells in type 2 diabetes mellitus by restoring mitophagy and alleviating endoplasmic reticulum stress. Adv. Sci. (Weinh). 10(19), e2300880 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leoncini, S. et al. Oxidative stress, erythrocyte ageing and plasma non-protein-bound iron in diabetic patients. Free Radic. Res.42(8), 716–724 (2008). [DOI] [PubMed] [Google Scholar]
- 49.León-Reyes, G. et al. Oxidative modifications of foetal LDL-c and HDL-c lipoproteins in preeclampsia. Lipids Health Dis.17(1), 110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ascaso, J. F. et al. Lipoprotein phenotype and insulin resistance in familial combined hyperlipidemia. Metabolism49(12), 1627–1631 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Cicero, A. F. et al. Short-term effects of a combined nutraceutical of insulin-sensitivity, lipid level and indexes of liver steatosis: A double-blind, randomized, cross-over clinical trial. Nutr. J.14, 30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu, W. et al. Major lipids and lipoprotein levels and risk of blood pressure elevation: A Mendelian randomisation study. EBioMedicine100, 104964 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveri, A. et al. Comprehensive genetic study of the insulin resistance marker TG:HDL-C in the UK Biobank. Nat. Genet.56(2), 212–221 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moran, C. et al. Dyslipidemia, insulin resistance, ectopic lipid accumulation, and vascular function in resistance to thyroid hormone β. J. Clin. Endocrinol. Metab.106(5), e2005–e2014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedreño, J. et al. Platelet function in patients with familial hypertriglyceridemia: Evidence that platelet reactivity is modulated by apolipoprotein E content of very-low-density lipoprotein particles. Metabolism49(7), 942–949 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. For further inquiries, please contact the corresponding author, Jianpeng Du, at 13811518062@163.com.