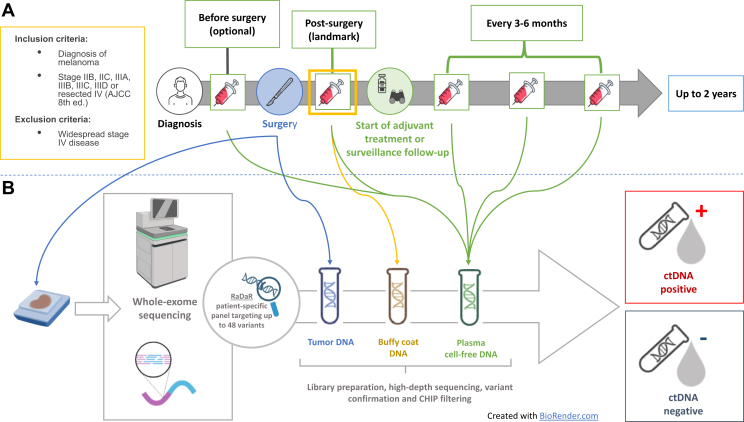
Figure 1.
The Serial ctDNA Monitoring as a Predictive Biomarker in Advanced NeoplAsms (SAMBA) study schema. (A) Patients with high-risk resected stage IIB-IV resected melanoma undergoing adjuvant treatment or surveillance were eligible for the study. Blood samples were collected at landmark (within 3 months from the completion of local treatment) and then every 3-6 months around the time of radiological restaging for up to 2 years. Additional plasma samples were collected before surgery and at progression, when feasible. (B) Archival tumor tissue was collected from all the patients and analyzed with whole-exome sequencing to identify somatic mutations and design patient-specific ctDNA panel. Longitudinal plasma samples were analyzed with the RaDaR assay. ctDNA, circulating tumor DNA.