Abstract
Individuals with human immunodeficiency virus (HIV) infection are prone to developing persistent and unremitting anogenital warts (AGWs). To address this health condition, immunotherapy using tuberculin purified protein derivative (PPD) has been proven to be a promising novel and safe treatment for AGWs in HIV patients. Therefore, this study involved a case of a 21-year-old man with stage I HIV infection, possessing CD4 counts 548 cells/uL and routinely receiving HIV antiretroviral. Patients presented with a condyloma acuminata type AGWs, supported by histopathological results, and tested positive for Human papillomavirus type 11. The treatment approach involved administering 15 tuberculin units of PPD weekly to the largest lesion for 5 weeks. The lesion started to respond two weeks following the first injection and showed a 50% decrease in size after five weeks. There was pain on the site of injection and sub-febrile for a short period. In conclusion, tuberculin PPD is safe and has the potential for the treatment of AGWs in HIV-positive patients for further development.
Key words: anogenital warts, purified protein derivative, immunotherapy, human papillomavirus, human immunodeficiency virus
Introduction
Anogenital warts (AGWs) are benign epithelial growths, which can appear on the genitalia, anus, or perianal area.1 AGWs are often florid and challenging to treat in human immunodeficiency virus (HIV)-positive individuals due to immunosuppression impairing the clearance of Human papilloma virus HPV infection.2 Furthermore, the treatment efficacy varies and is often followed by recurrence. Among various therapeutic modalities, immunotherapy emerges as a promising approach to stimulate systemic immune response, capable of treating distant lesions and offering long-term prevention of recurrence.3 This approach also produces minimal side effects, with a success rate of 45%–75% for various cases of verrucae and AGWs.4 Tuberculin purified protein derivative (PPD) is a safe option for individuals with HIV infection, as it only contains Mycobacterium tuberculosis (M. tuberculosis) antigen without being a complete pathogen.5 The mechanism involves a delayed-type hypersensitivity reaction against virus particles at injection sites and other locations, leading to the complete and safe eradication of warts.6,7 Several investigations and case reports have highlighted the efficacy and safety of PPD as the treatment for anogenital and extragenital warts,3,4,6,7 with successful rates ranging from 45% to 60%.4 However, there are no reports on the use of PPD for the treatment of AGWs in HIV patients. This study presents a case involving perianal AGWs in an individual with HIV infection who was treated using intralesional injection of tuberculin PPD.
Case Report
A 21-year-old man who had male-to-male sexual activity presented with mild itch and multiple skin-colored papules on the perianal area. Patient was HIV-positive and had been consistently taking antiretroviral medication including tenofovir, lamivudine, and dolutegravir for 10 months. There was no history of any symptoms related to sexually transmitted infections (STI). The initial lesion emerged as a single skin-colored papule on the perianal area, which grew larger and multiplied. Patient was diagnosed as AGWs and had previously received two sessions of cryotherapy, which resulted in only a minor improvement with unpleasant side effects. Physical examination showed multiple, non-tender, 0.5x0.5x0.3 cm to 2x1.5x0.5 cm in size, verrucous papules, and tumors around the perianal verge (Figure 1a). Meanwhile, the histopathologic result showed hyperplasia with koilocytes, which supported the diagnosis of AGWs (Figure 2a-d). Patient was treated with five doses of PPD-15TU (tuberculin unit) per week for five sessions, injected intralesionally after previously tested by tuberculin skin test, which yielded a 15 mm diameter of induration. Patient first complained of pain at the injection site but quickly subsided without the use of analgesics within 15 minutes. There was a complaint of sub febrile 12 hours after tuberculin PPD injection which subsided within 24 hours. The area where the injection was administered showed no symptoms of swelliness, nodules, blisters, or abscesses. Although some of the AGWs lesions had a 50% regression in size on the 21st day after the initial injection (Figure 1c). There was also a new skin lesion on the 14th day after the fifth dose of PPD-15TU injection. Patient expressed satisfaction with the current improvement and decided to observe the progress of the lesion without further treatment due to the increased workload. Based on telephone communication, at one month of follow-up after the last visit, the lesion fully diminished without additional side effect, and 11 months of follow-up.
Discussion
In individuals with HIV infection, AGW often tends to exhibit larger or more numerous lesions, a poor response to therapy, and a higher recurrence rate after therapy.8 Highly active antiretroviral therapy (HAART) which has been running for less than 5 years has no significant effect on HPV eradication.9 In this study, the absence of spontaneous resolution of AGWs lesions in patients could be caused by an immunocompromised condition due to HIV infection, and the under 5 years duration of HAART.
A dermoscopy of genital warts showed mosaic, finger-like, and knob-like patterns, while the disappearance of vascular elements indicated the complete resolution of a given lesion of warts.10 The skin lesions around the anal area in patients in this case report aligned with the clinical presentation of AGWs of the condyloma acuminata type in the form of a mucosa-colored tumor with a verrucous surface, resembling a cauliflower. The fingerlike dermoscopic appearance obtained at each observation supported the diagnosis of AGWs. Furthermore, four weeks after the first tuberculin PPD injection, the vascular structure was reduced, confirming the resolution process. The histopathological features of AGWs commonly show hyperkeratosis, parakeratosis, hypergranulosis, basal cell hyperplasia, and papillomatosis. In the stratum granulosum, koilocytes can also be found representing the cytopathic effect of HPV.11 In this case report, the histopathological features supported the diagnosis of AGWs.
AGWs can be treated through various approaches, including surgical procedures such as electrocautery, excision surgery, cryotherapy, and laser therapy, as well as non-surgical approach, namely podophyllin, podophyllotoxins, trichloroacetic acid, and immunotherapy.6 Several negative effects of therapy had been documented, including discomfort, burning, crusting, ulceration, infection, and permanent scarring, making the approach less suitable for individuals with multiple warts or distant lesions.3,6 According to the definition, immunotherapy was found to be a biological therapy that involves the use of agents for activating or suppressing the immune system to help the body fight cancer, infection, and other diseases.12 Previous investigations had hypothesized that immunotherapy was related to the release of many proinflammatory cytokines, strengthening the immune response against HPV,13 in both the treated area and distant sites.14 The cytokines Interferon (IFN)-γ and interleukin (IL)-12, exhibiting a T-helper 1 pattern, are released when tuberculin PPD stimulates a robust systemic immune response, activating cytotoxic T cells and natural killer (NK) cells to fight HPV infection.6,7 Compared to cryotherapy (18%), the PPD group exhibited significantly greater rates of complete remission (77%) and less negative side effects, such as hypertrophic scarring.3 Therefore, immunotherapy was recommended for recalcitrant, recurrent HPV infection, and lesions occurring in hard-to-reach areas, or extensive skin lesions.12 Due to the large size of the skin lesions, immunotherapy was preferred for patients in this case report.
Figure 1.
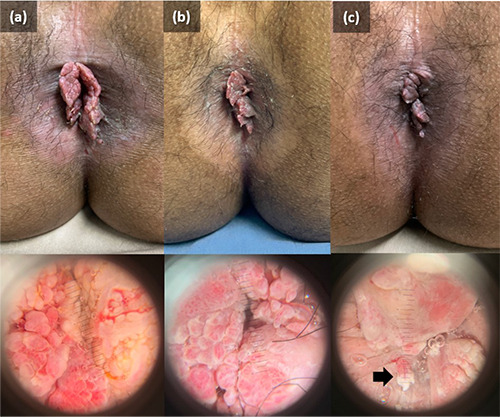
Clinical manifestation and dermoscopic features of anogenital warts on perianal area before (a), after the third injection (b) and after the fifth injection (c) of tuberculin PPD. Anogenital warts lesions had partial with 50% regression in size after the last injection of PPD-15TU, with new skin lesion appeared (black arrow).
Although various immunotherapy is available for AGWs including the use of live vaccine attenuated vaccine the Bacillus Camette-Guerin vaccine, its application in immunodeficiency conditions such as HIV is contraindicated.15 Tuberculin PPD is a protein extracted from M. tuberculosis cultures and is generally used to assess the history of exposure to tuberculin protein, due to vaccination or infection.5 In this study, patients was treated with tuberculin PPD RT 23 in a 1 milliliter (ml) vial, containing a composition of 0.4 mcg tuberculin PPD RT 23 from M. tuberculosis, 7.6 mcg disodium phosphate dihydrate, 1.45 mcg potassium dihydrogen phosphate, 4.8 mcg sodium chloride, 100 mcg potassium hydroxyquinoline sulfate, 50 mcg polysorbate 80, and 1 ml water.16 Tuberculin PPD was found safe for individuals with HIV infection, as it only contained M. tuberculosis antigen, an incomplete pathogen. Meanwhile, contraindications encompassed severe reactions to previous tuberculin tests including necrosis, formation of blisters or ulcers at the injection site, and anaphylactic shock.5 Regarding the highest dose of tuberculin PPD, the administration of 88 TU was found safe and the typical induration resulting from tuberculin test resolved within 5–10 days,17 leading to the selection of 7-day interval between each injection in this case report.
Immunotherapy using intralesional tuberculin PPD injection for AGWs is a minimally invasive, secure, and affordable mode of treatment, specifically with multiple or resistant warts, with positive outcomes reported in some studies.14,4,8 Azab et al. reported that AGWs patients had complete resolution within 3 months after intralesional injection of tuberculin PPD at a dose of 10 TU every two weeks for six sessions, showing a significantly high IL-17 serum level (p<0.05) associated with a clinical response to tuberculin PPD. Localized discomfort at the injection site, edema, systemic symptoms, erythema, swelling, and hypo- or hyperpigmentation, following an inflammatory reaction, were the most frequently reported side effects.14 Similarly, Mehta et al. found that tuberculin PPD therapy for facial warts resulted in complete resolution in 45% of subjects, partial resolution in 37%, and no response in 18.4%. The assessment was carried out two weeks after intradermal injection of tuberculin PPD at a dose of 10 TU in the volar region of the forearm, which was repeated every two weeks for five sessions or until a complete resolution was achieved. The side effects that occurred included pain, erythema, and edema at the injection site for 2–3 days, without causing therapy discontinuation.4 In this study, patients experienced a mild side effect in the form of sub febrile, which occurred 12 hours after tuberculin PPD injection, and subsided within 24 hours. Other side effects reported were pain at the injection site, without causing discontinuation of therapy.
Figure 2.
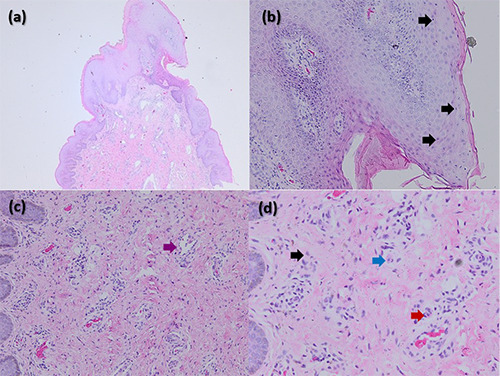
Histopathological features of anogenital warts lesions; epitelial hyperplasia (a); koilocytes in the epidermis (b); vasodilatation in fibrocollagen layer (c); inflammatory cells infiltration that consists of lymphocyte (black arrow), plasma cell (blue arrow), and neutrophils (red arrow) (d).
Several studies have been conducted to compare the efficiency of tuberculin PPD with other immunotherapy drugs on AGWs and common warts. Abou-Taleb et al. compared effectiveness of intralesional vitamin D3 injections of 200,000 IU with tuberculin PPD administered every three weeks for three sessions, or until complete remission was obtained. Meanwhile, complete resolution accompanied by significantly higher serum levels of IL-12 and IFN-γ (p=0.034 and p=0.04) was found in 59.1% of the PPD group compared to 21.7% in the vitamin D group.18 Shaheen et al. also compared the efficacy of tuberculin PPD and the mumps measles as well as rubella (MMR) vaccine and tuberculin PPD administered intralesionally in the treatment of common warts with multiple AGWs. The result showed that therapy with tuberculin PPD resulted in 60% local and systemic response, while the MMR vaccine yielded 80% local and 40% systemic response.19 In this case report, effectiveness of therapy was evaluated by measuring the volume of the lesion quantitatively from the sum of the multiplication of the length, width, and thickness of each lesion. During observation, there was a 50% regression of AGWs lesions on day 21 after initiation of therapy. There was new lesion growth appeared on the 14th day of observation after immunotherapy with 15 TU tuberculin PPD had been given 5 times, but on one month after the last visit, the lesion was completely diminished, and at 11 months of follow-up, there was no sign of recurrence.
Conclusions
In conclusion, this study showed that treating AGWs in HIV patients with tuberculin PPD is a safe and well-tolerated procedure with promising results. Moreover, further investigation is recommended to advance the application of tuberculin PPD for AGWs in HIV patients.
Acknowledgments
The authors are grateful to the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
References
- 1.Gilson R, Nugent D, Werner RN, et al. 2019 IUSTI-Europe guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2020;34:1644-53. [DOI] [PubMed] [Google Scholar]
- 2.Chikandiwa A, Pisa PT, et al. Incidence, persistence, clearance, and correlates of genital human papillomavirus infection and anogenital warts in a cohort of men living with human immunodeficiency virus in south africa. Sex Transm Dis. 2019;46:347-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldahan AS, Mlacker S, Shah V V, et al. Efficacy of intralesional immunotherapy for the treatment of warts: A review of the literature. Dermatol Ther. 2016;29:197-207. [DOI] [PubMed] [Google Scholar]
- 4.Mehta KS, Chauhan PS, Chandel M, et al. Evaluation of efficacy and safety of intradermal ppd for treating facial warts - a prospective study. IJCED-IP. 2019;5:322-6 [Google Scholar]
- 5.Pahal P, Pollard EJ, Sharma S. PPD Skin Test. [Updated 2023 Apr 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556037/. [PubMed] [Google Scholar]
- 6.Achdiat PA, Antariksa NC, Rowawi R, et al. Success of intralesional purified protein derivative immunotherapy in the treatment of anogenital warts: A case report. J Exp Pharmacol. 2022;14:131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suwarsa O, Achdiat PA, Rowawi R, et al. Effect of Purified Protein Derivative Therapy on Serum Interleukin-12 Levels in Anogenital Warts Patients. Media Dermato Venereologica Indonesiana. 2022;49:190-219. [Google Scholar]
- 8.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidalgo-Tenorio C, Gil-Anguita C, López Ruz MA, et al. ART is key to clearing oncogenic HPV genotypes (HR-LPV) in anal mucosa of HIV-positive MSM. PLoS One. 2019;14:e0224183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankad B, Koti V. Dermoscopic assessment of periungual and genital warts treated by multiple modalities: A report. Int J Res Dermatol. 2022;8:415-8. [Google Scholar]
- 11.Winer RL, Koutsky LA. Genital human papillomavirus infection. In: Holmes HK, Sparling PF, Stamm WE, Piot P, et al., editors. Sexually transmitted diseases. 4th Ed. United States of America: McGraw Hill; 2008. [Google Scholar]
- 12.Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaheen MA, Salem SA, Fouad DA, et al. Intralesional tuberculin (PPD versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194-200. [DOI] [PubMed] [Google Scholar]
- 14.Azab M, El-Shabrawy MM, Nafea EROA, et al. Measurement of serum interleukin 17 level in patients with genital warts before and after intralesional tuberculin injection. J Mens Health. 2022;18:14. [Google Scholar]
- 15.Achdiat PA, Suwarsa O, Hidayat YM, et al. Succesful treatment of anogenital warts with single dose Bacillus Calmette Guerin vaccine without prior sensitization in tuberculosis endemic country: Two case report. Hum Vaccin Immunother. 2023;19:2187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biofarma. Tuberculin PPD RT 23 SSI (2 TU). [Updated 2017 Nov 13]. Accessed 2023 August 13. Available from: https://www.biofarma.co.id/en/latest-news/detail/tuberculinppd-rt-23-ssi-2-tu. [Google Scholar]
- 17.Saoji V, Lade NR, Gadegone R, et al. Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016;82:42-6. [DOI] [PubMed] [Google Scholar]
- 18.Aboutaleb D, Abou-Taleb H, El-Badawy O, et al. Intralesional vitamin D3 versus intralesional purified protein derivative (PPD) in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2019;32:e13034. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen MA, Salem SA, Fouad DA, et al. Intralesional tuberculin (ppd) versus measles, mumps, rubella (mmr) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2015;28:194-200. [DOI] [PubMed] [Google Scholar]


