Abstract
Pigmented epithelioid melanocytoma (PEM) is a rare and diagnostically challenging skin neoplasm. Differentiating this lesion from melanoma is challenging due to overlapping clinical, histopathological, and immunohistochemical features. Some PEMs have a benign course, while others may be associated with more aggressive behaviour and have potential for local recurrence or distant metastasis. So, careful evaluation and follow-up are crucial for individuals with a diagnosis of PEM. This case study focuses on a 42-year-old woman with a gradually enlarging, dark, pigmented gluteal lesion. Histopathological analysis following complete excision showed nodular proliferation of spindled and epithelioid melanocytes in the dermis, extending into subcutaneous fat. Cellular atypia or mitotic figures were absent, confirming the diagnosis as PEM. This case underscores the intricate balance between monitoring and intervention in complex dermatological cases, shedding light on diagnostic challenges and ultimately striving for improved patient care.
Keywords: Melanocytes, melanoma, skin
Introduction
Pigmented epithelioid melanocytoma (PEM), previously referred to as ‘Animal type melanoma’, is a rare and intricate neoplasm that presents a significant diagnostic conundrum. This cutaneous tumour is characterized by cellular features, often leading to misdiagnosis due to its overlap with malignant melanoma. This case report sheds light on a recent encounter with PEM, contributing to the understanding of its clinical manifestations, diagnostic complexities, and treatment considerations.[1]
Case Report
The focal point of this case is a 42-year-old female patient who brings to fore the importance of recognizing PEM as a distinct entity. She presented with a two-year history of an asymptomatic, dark, and uniformly pigmented lesion on her gluteal region, gradually increasing in size. The patient’s medical history was unremarkable, devoid of familial melanoma instances, or excessive exposure to sunlight or artificial tanning sources. Clinical examination revealed an elevated, solid, darkly pigmented lesion with regular borders, measuring 2.5 × 2 cms [Figure 1]. To unravel the mysteries of this lesion, a complete excisional biopsy was performed.
Figure 1.
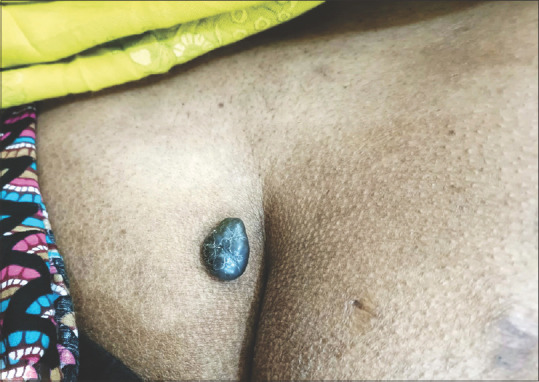
Clinical picture showing an elevated, darkly pigmented lesion with regular borders in gluteal region
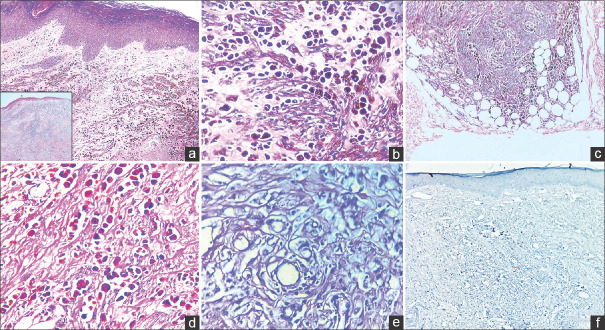
Histopathological examination disclosed a nodular proliferation of spindled and epithelioid melanocytes in the dermis which displayed edema. These cells exhibited abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli [Figure 2]. They were seen extending from the superficial dermis into the subcutaneous fat, reaching the deep resected margin. No cellular atypia/mitotic figures were seen. Ki67 was low (<1%). The diagnosis was established as PEM, warranting further assessment of the lesion’s potential for aggressive behavior. This case underscores the importance of cautious observation when confronted with such intricate dermatological challenges.
Figure 2.
a: Histopathology image disclosing nodular proliferation of spindled and epithelioid pigmented melanocytes (H & E,40x). Inset: Low-power silhouette of tumor; b: The tumor cells are heavily pigmented and have abundant eosinophilic cytoplasm, vesicular nuclei and prominent nucleoli (H & E,400x); c: The tumor cells are seen extending to the subcutaneous fat (H & E,100x); d: PAS-D stain negative for mucin (400x); e: Melanin bleached H & E image (400x); f: Ki-67 IHC stain displaying low proliferative index (<1%) in the tumor proper (100x)
Discussion
Distinguishing between PEM and melanoma presents a formidable task due to their overlapping clinical, histopathological, and immunohistochemical (IHC) features. The distinguishing characteristics that help unravel the diagnostic puzzle are described [Table 1].[2,3] The characteristic clinical manifestations featuring a slowly progressing tumour and a clear histopathological profile depicting pigmented epithelioid cells without atypia, a low mitotic rate, and absence of lymphovascular invasion (LVI) played a pivotal role in confidently confirming the diagnosis in our case and robustly excluded the possibility of melanoma.[4]
Table 1.
Distinguishing features of Pigmented Epithelioid Melanocytoma (PEM) and Melanoma
| Sl no. | Key features | PEM | Melanoma |
|---|---|---|---|
| 1. | Clinical presentation | ||
| a. | Lesion appearance | Presents as a darkly pigmented, well-circumscribed lesion with variable coloration, including shades of brown, black, gray, and sometimes blue. | Presents as a darkly pigmented, asymmetrical lesion with irregular borders and variable coloration with shades of brown and black. They are usually evolving lesions which change in size, shape or color |
| b. | Size and Growth | Typically smaller in size and tend to grow slowly over time | Can vary in size and often exhibit rapid growth. |
| 2. | Histopathology: | ||
| a. | Cellular Architecture | Proliferation of epithelioid melanocytes with abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. No atypia/pleomorphism noted. | May show a wider range of cytologic atypia and pleomorphism. |
| b. | Mitotic Activity | Lower mitotic rate | Usually higher mitotic rate |
| c. | Pagetoid Spread | Less common | Often display pagetoid (single cell) spread within the epidermis |
| d. | Junctional activity | Less common | More common |
| e. | Lymphovascular Invasion | Less common | More common |
| 3. | Immunohistochemistry | ||
| a. | S100 | Both PEM and melanoma cells may stain positive for S100 protein, but PEM cells typically show a more diffuse and strong staining pattern. | |
| b. | HMB-45 | Positive staining for HMB-45 is common in both PEM and melanoma, but PEM cells may show a more consistent and diffuse staining | |
| c. | SOX10 | Positive staining for SOX10 is often seen in both PEM and melanoma, with stronger and diffuse staining in PEM. | |
| 4. | Genetic and Molecular Features: | ||
| a. | BRAF Mutation | Less frequent | More common |
| b. | KIT Mutations | Some cases have been associated with KIT mutations | Less common |
| c. | Cytogenetic Abnormalities | Less prevalent | More prevalent in melanomas |
| 5. | Clinical Behaviour | ||
| a. | Metastasis | Less aggressive and can metastasize | More aggressive and have a higher potential for metastasis |
| b. | Prognosis | Indolent clinical course with good prognosis | Poor prognosis |
PEMs are primarily found in children, adolescents, and young adults, although occurrences can manifest across various age groups. This age predilection is a differentiating factor from conventional melanoma, which often appears in older individuals. These lesions most commonly occur on the trunk and extremities, but instances on mucous membranes have also been documented.[5] It is also crucial to distinguish PEM from other benign conditions that can mimic it, such as blue nevus/cellular blue nevus, dendritic blue nevus, and deep penetrating nevus (DPN). In cellular blue nevus, cells are typically non-pigmented and their nuclei do not exhibit an epithelioid appearance. Distinct features like dermal sclerosis and the diffuse dermal component instead of a nested one helps differentiate dendritic blue nevus from PEM. The primary distinction between DPN and PEM is in their histological patterns. DPNs have a biphasic pattern with a unique junctional component and infiltration into the dermis, while PEMs are characterized by sheets of heavily pigmented epithelioid melanocytes.[2]
IHC findings may overlap in these lesions with S100, Melan A, and HMB45 positivity. High Ki-67 labeling indices are associated with increased cellular proliferation in PEM and may suggest a more aggressive behavior. IHC showing loss of cytoplasmic expression of PRKAR1A in tumour cells is a specific alteration seen in some cases of PEM. Molecular alterations such as GNAQ or GNA11 are observed in blue nevus and dendritic blue nevus, while DPN displays mutations in BRAF, MAP2K1, and CTNNB1. PEM lesions may exhibit alterations in PRKCA, PRKAR1A, GNAQ, and MAP2K1.[2] FISH (Fluorescence In Situ Hybridization) plays an important role in identifying these genetic alterations. In resource-poor settings, the decision to perform these ancillary studies should be based on clinical suspicion, histopathology findings, available expertise, and resources. While these studies can enhance diagnostic accuracy, their absence does not necessarily preclude a correct diagnosis. However, in cases with atypical or ambiguous histological findings, ancillary studies become increasingly important for confirming diagnosis and ruling out other melanocytic neoplasms.[2]
The treatment approach for PEM primarily involves surgical excision. Wide local excision with clear margins is a preferred method, which ensures complete removal of lesion. Given the low malignant potential of PEM, surgical excision is typically curative. In most cases, once adequately excised, PEM does not necessitate further adjuvant treatments such as chemotherapy or radiation therapy. However, long-term follow-up is recommended to monitor for any signs of recurrence or metastasis.[1]
Conclusion
The patient’s journey from presentation of a pigmented lesion to eventual diagnosis of PEM underscores the intricate interplay between clinical observation, histopathological analysis, and specialized expertise. This case underscores an indispensable role that collaboration between clinicians and pathologists plays in deciphering diagnostic intricacies and ensuring optimal patient outcomes. This case report functions as a guiding light toward enhanced understanding, heightened diagnostic accuracy, and ultimately improved patient care.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gavriilidis P, Michalopoulou I, Chatzikakidou K, Nikolaidou A. Pigmented epithelioid melanocytoma: A new concept encompassing animal-type melanoma and epithelioid blue nevus. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-008865. bcr-2013-008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayraktar EC, Jour G. Pigmented epithelioid melanocytomas and their mimics;Focus on their novel molecular findings. Biology (Basel) 2021;10:1290. doi: 10.3390/biology10121290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramondetta A, Ribero S, Conti L, Quaglino P, Broganelli P. A case of a pigmented epithelioid melanocytoma on a mucosal site. Dermatol Pract Concept. 2020;10:e2020070. doi: 10.5826/dpc.1004a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thielmann CM, Ugurel S, Livingstone E, Zimmer L, Paredes BE, Brinkmeier T, et al. Metastatic pigmented epithelioid melanocytoma in a 7-year-old female. J Dtsch Dermatol Ges. 2021;19:1217–9. doi: 10.1111/ddg.14523. [DOI] [PubMed] [Google Scholar]
- 5.Cheng PS, Chuang SS, Kuo TT, Lai FJ. Pigmented epithelioid melanocytoma: Report of a case and review of 173 cases in the literature. Dermatol Sin. 2012;30:57–61. [Google Scholar]