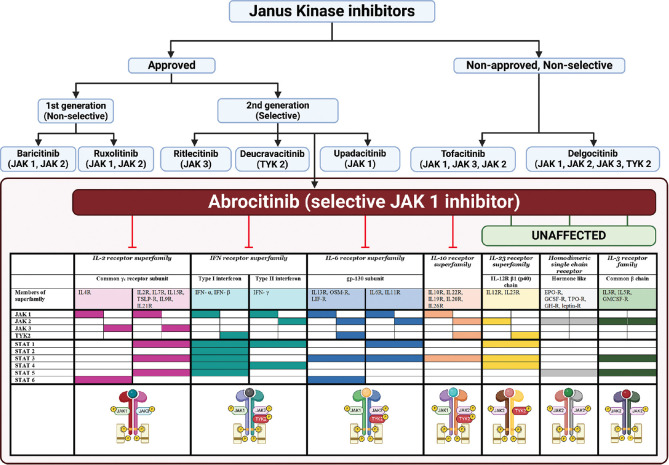
Figure 1.
Janus Kinase inhibitors in dermatology: Selective inhibition of JAK1 by abrocitinib. “Created with BioRender.com”. JAK inhibitors can be classified as first-generation (non-selective) and second-generation (selective), depending upon the JAK(s) they inhibit. (top) JAK inhibitors approved by the US-FDA in dermatology for use include: first generation- baricitinib (alopecia areata) and ruxolitinib (vitiligo); and among second generation- ritlecitinib (alopecia areata), deucravacitinib (psoriasis), Upadacitinib, and abrocitinib (atopic dermatitis). Abrocitinib (bottom) is a selective JAK1 inhibitor that inhibits various downstream mediators in atopic dermatitis, as depicted. All are systemic molecules, except ruxolitinib which is US-FDA approved for topical use in vitiligo, and delgocitinib which is approved in Japan for topical use in atopic dermatitis