Abstract
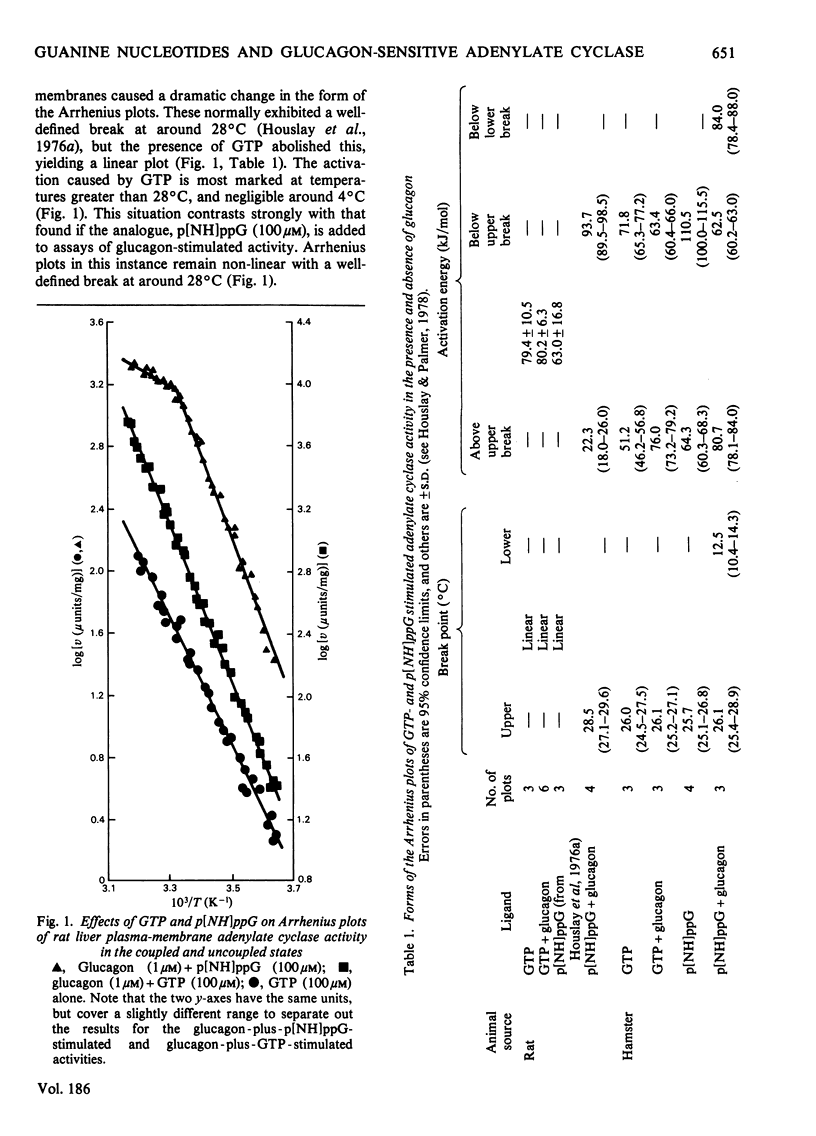
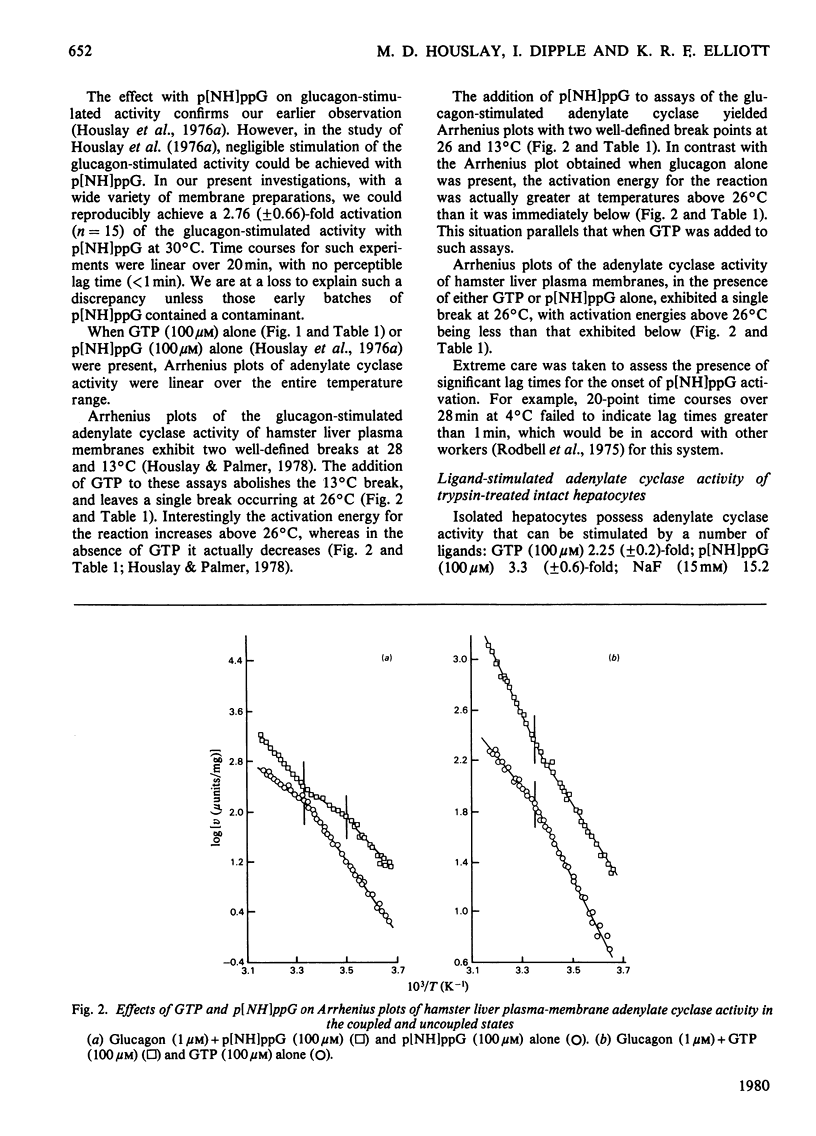
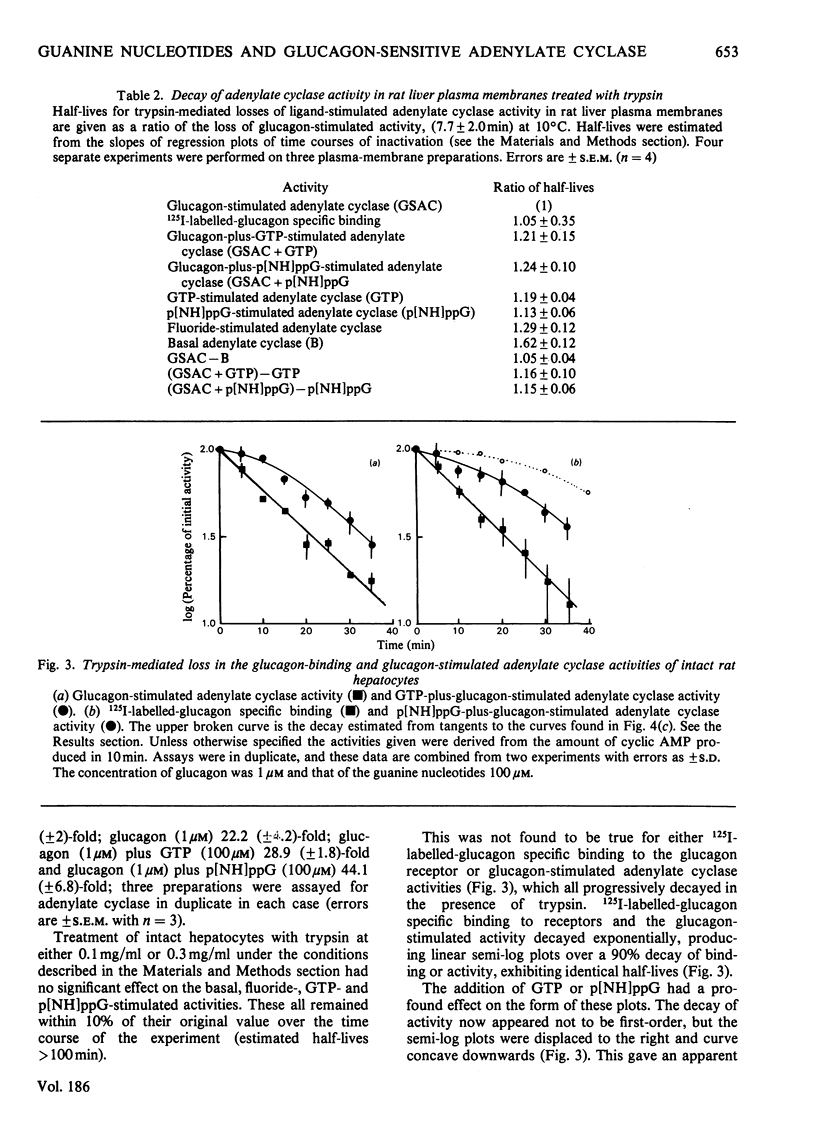
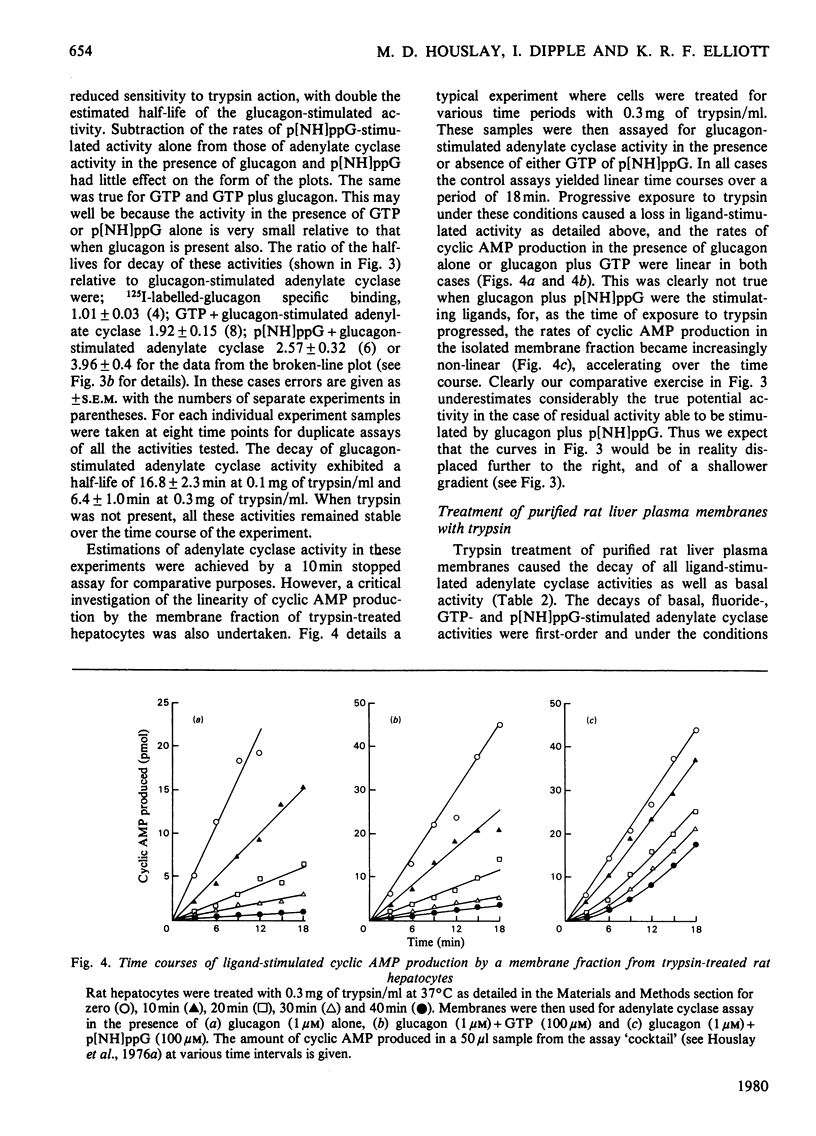
1. GTP, but not p[NH]ppG (guanosine 5′-[βγ-imido]triphosphate), abolishes the sensitivity of glucagon-stimulated adenylate cyclase to the lipid-phase separations occurring in the outer half of the bilayer in liver plasma membranes from rat. 2. When either GTP or p[NH]ppG alone stimulate adenylate cyclase, the enzyme senses only those lipid-phase separations occurring in the inner half of the bilayer. 3. Trypsin treatment of intact hepatocytes has no effect on the basal, fluoride-, GTP- or p[NH]ppG-stimulated adenylate cyclase activity. However, 125I-labelled-glucagon specific binding decays with a half-life matching that of the decay of glucagon-stimulated adenylate cyclase activity. 4. When GTP or p[NH]ppG are added to assays of glucagon-stimulated activity, the half-life of the trypsin-mediated decay of activity is substantially increased and the decay plots are no longer first-order. 5. Trypsin treatment of purified rat liver plasma membranes abolishes basal and all ligand-stimulated adenylate cyclase activity, and 125I-labelled-glucagon specific binding. 6. Benzyl alcohol activates the GTP- and p[NH]ppG-stimulated activities in an identical fashion, whereas these activities are affected differently when glucagon is present in the assays. 7. We suggest that guanine nucleotides alter the mode of coupling between the receptor and catalytic unit. In the presence of glucagon and GTP, a complex of receptor, catalytic unit and nucleotide regulatory protein occurs as a transient intermediate, releasing a free unstable active catalytic unit. In the presence of p[NH]ppG and glucagon, the transient complex yields a relatively stable complex of the catalytic unit associated with a p[NH]ppG-bound nucleotide-regulatory protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L. Hormone-sensitive adenylyl cyclases. Useful models for studying hormone receptor functions in cell-free systems. Biochim Biophys Acta. 1973 Sep 10;300(2):129–158. doi: 10.1016/0304-4157(73)90002-6. [DOI] [PubMed] [Google Scholar]
- Craik J. D., Elliott K. R. Kinetics of 3-O-methyl-D-glucose transport in isolated rat hepatocytes. Biochem J. 1979 Aug 15;182(2):503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipple I., Houslay M. D. The activity of glucagon-stimulated adenylate cyclase from rat liver plasma membranes is modulated by the fluidity of its lipid environment. Biochem J. 1978 Jul 15;174(1):179–190. doi: 10.1042/bj1740179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. Coupling of the glucagon receptor to adenylate cyclase. Biochem Soc Trans. 1979 Oct;7(5):843–846. doi: 10.1042/bst0070843. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Ellory J. C., Smith G. A., Hesketh T. R., Stein J. M., Warren G. B., Metcalfe J. C. Exchange of partners in glucagon receptor-adenylate cyclase complexes. Physical evidence for the independent, mobile receptor model. Biochim Biophys Acta. 1977 Jun 2;467(2):208–219. doi: 10.1016/0005-2736(77)90197-3. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. The lipid environment of the glucagon receptor regulates adenylate cyclase activity. Biochim Biophys Acta. 1976 Jun 17;436(2):495–504. doi: 10.1016/0005-2736(76)90211-x. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Palmer R. W. Changes in the form of Arrhenius plots of the activity of glucagon-stimulated adenylate cyclase and other hamster liver plasma-membrane enzymes occurring on hibernation. Biochem J. 1978 Sep 15;174(3):909–919. doi: 10.1042/bj1740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Palmer R. W. Lysophosphatidylcholines can modulate the activity of the glucagon-stimulated adenylate cyclase from rat liver plasma membranes. Biochem J. 1979 Jan 15;178(1):217–221. doi: 10.1042/bj1780217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Swartz T. L., Birnbaumer L. Coupling of glucagon receptor to adenylyl cyclase. Requirement of a receptor-related guanyl nucleotide binding site for coupling of receptor to the enzyme. J Biol Chem. 1979 Feb 25;254(4):1119–1123. [PubMed] [Google Scholar]
- Johnson R. A., Pilkis S. J., Hamet P. Liver membrane adenylate cyclase. Synergistic effects of anions on fluoride, glucagon, and guanyl nucleotide stimulation. J Biol Chem. 1975 Aug 25;250(16):6599–6607. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Sauerheber R. D., Gordon L. M., Crosland R. D., Kuwahara M. D. Spin-label studies on rat liver and heart plasma membranes: do probe-probe interactions interfere with the measurement of membrane properties? J Membr Biol. 1977 Feb 24;31(1-2):131–169. doi: 10.1007/BF01869402. [DOI] [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]


