Figure 4.
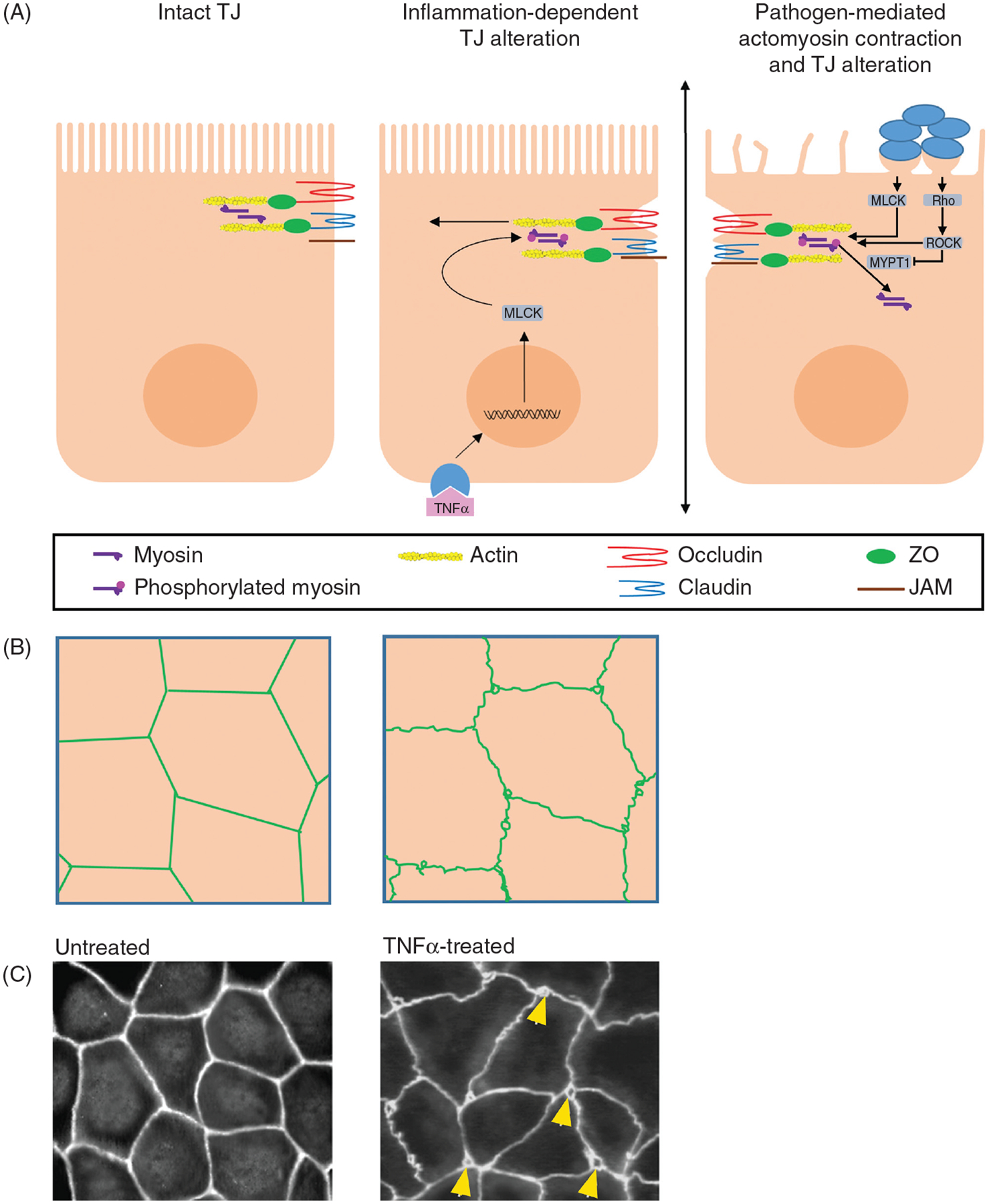
Pathogens may trigger contraction of the perijunctional actomyosin ring, resulting in increased paracellular permeability by the leak pathway. (A) Schematic, lateral view: Some pathogens trigger signaling pathways that activate myosin light chain kinase (MLCK) and/or Rho-associated protein kinase (ROCK), which can both phosphorylate myosin light chain and cause actomyosin contraction. ROCK also phosphorylates myosin phosphatase target subunit 1 (MYPT1) and, thereby, inhibits MLC dephosphorylation. Alternatively, proinflammatory molecules like TNFα can also activate MLCK. (B) Schematic, en face view: Uniform distribution of TJ proteins (green) preserved at the periphery of uninfected cells. Pathogens or proinflammatory molecules like TNFα induce actomyosin contraction leading to the distortion and opening of junctional areas. Gaps may also be evident at points of cell contacts. (C) Example: Uniform ZO-1 staining at cell periphery of cultured Caco-2 monolayers. On the right, TNFα-induced disruption of ZO-1 localization, and appearance of gaps (arrowhead) at points of cell contact. Image obtained, with permission, from Ma et al. (66).
